Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5910
Review Article(ISSN: 2638-5910) 
Tirzepatide as Anti-Obesity Agent Volume 4 - Issue 2
Nasser Mikhail1* and Soma Wali2
- 1Endocrinology Division, Olive View UCLA Medical Center, David Geffen UCLA School of Medicine, USA
- 2Department of Medicine, Olive View UCLA Medical Center, David Geffen UCLA School of Medicine, USA
Received: June 29, 2022 Published: July 11, 2022
Corresponding author: Nasser Mikhail, Endocrinology Division, Olive View UCLA Medical Center, David Geffen UCLA School of Medicine, USA, Sylmar, CA, USA
DOI: 10.32474/ADO.2022.04.000183
Abstract
Tirzepatide was recently approved in the US for treatment of type 2 diabetes. This agent is currently under investigation for use as anti-obesity agent in individuals without diabetes. In a large phase 3 clinical trial, mean percentage reductions in body weight were 15.0%, 19.5%, 20.9%, and 3.1% with weekly subcutaneous injections of tirzepatide 5 mg, 10 mg, 15 mg, and placebo at 72 weeks, respectively (P<0.001 for all comparisons with placebo). This weight loss resulted in favorable effects on blood pressure, lipid profile, and plasma levels of glucose and insulin. In addition, 95.3% of terzipatide-treated participants with pre-diabetes at baseline reverted to normoglycemia at 72 weeks compared with 61.9% of subjects in the placebo group. Gastrointestinal (GI) adverse effects were the commonest reported symptoms associated with tirzepatide use. Hypoglycemia (blood glucose <54 mg/dl) was more frequent with tirzepatide (1.4-1.6%) versus placebo (0.2%). Drug discontinuation rates due to adverse effects were 4.3-7.1% and 2.6% in the tirzepatide and placebo groups, respectively. Overall, tirzepatide is a promising potent anti-obesity agent. Further studies are needed to determine its long-term safety and efficacy.
Keywords: Tirzepatide; Semaglutide; Obesity; Efficacy; Safety; Weight loss
Introduction
Tirzepatide (LY3298176) is a dual receptor agonist of the 2 incretins: glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) [1]. Its 39 amino acid sequence is mainly based on that of native GIP which consists of 42 amino acids [1]. Tirzepatide is attached to a 20-carbon fatty diacid moiety which binds to albumin. Albumin binding prolongs its half-life to approximately 5 days allowing once a week subcutaneous administration [2]. Tirzepatide has similar GIP receptor binding affinity to native GIP. Yet, it has 5 times lower affinity to GLP- 1 receptor compared to native GLP-1 [2]. Thus, this drug was described as an imbalanced dual agonist in favor of GIP receptor over GLP-1 receptor activity [3]. Tirzepatide (Mounjaro) was recently approved by the Food and Drug Administration (FDA) in May 2022 for treatment of adults with type 2 diabetes based on results of a series of phase 3 clinical trials (SURPASS-1 through 5) [4,5]. In the SURPASS trials, tirzepatide use was associated with potent dose-related effect on weight loss. Recently, a landmark study called SURMOUNT-1 was published to specifically evaluate tirzepatide as anti-obesity drug in subjects without diabetes [6]. The purpose of this article is to provide an appraisal of tirzepatide as a new potential therapeutic agent for obesity.
The SURMOUNT-1 Trial
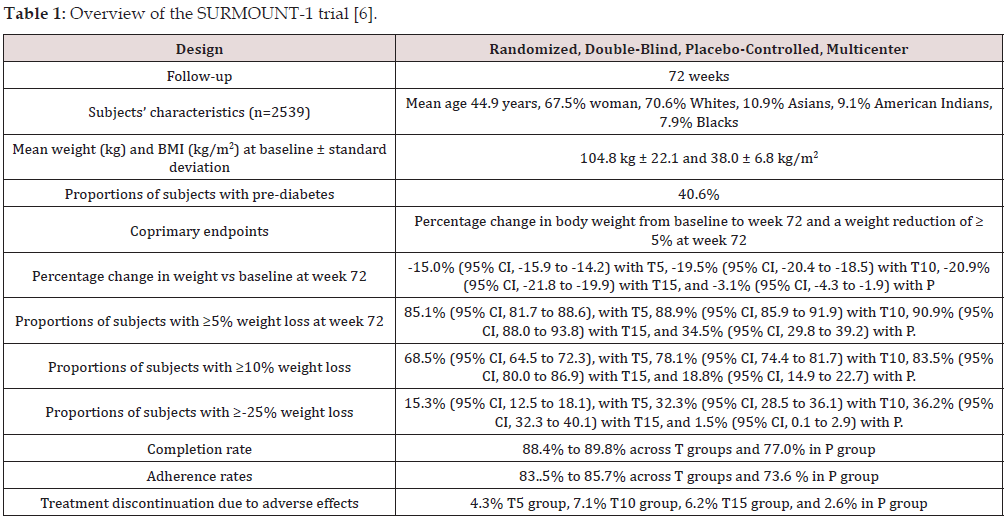
The SURMOUNT-1 trial was a phase-3 double-blind, placebocontrolled, randomized trial including 2,539 obese adults without diabetes. At baseline, the mean body weight was 104.8 kg and mean body mass index (BMI was 38.0 kg/m2) [6]. The study consisted of 4 groups of subjects randomized to once-weekly 5 mg, 10 mg, 15 mg of tirzepatide given subcutaneously and a fourth placebo group. All participants received lifestyle intervention. Coprimary endpoints were the percentage change in weight from baseline and a weight reduction of 5% or more. At week 72, the placebo-adjusted mean percentage change in weight was -11.9% (95% CI -13.4 to -10.4) for the 5-mg dose, -16.4% (95% CI, -17.9 to -14.8) for the 10-mg dose, and -17.8% (95% CI, -19.3 to -16.3) for the 15-mg dose (P<0.001 vs placebo) [6]. Regarding the second component of primary endpoint, 85% (95% CI, 82 to 89), 89% (95% CI, 86 to 92), and 91% (95% CI, 88 to 94) of participants in the 5-mg, 10-mg, and 15-mg tirzepatide groups, respectively, had a body weight reduction of 5% or more at 72 weeks compared with 35% (95% CI, 30 to 39) of participants in the placebo group [6]. Weight loss with tirzepatide treatment started within the first 8 weeks of use, increases progressively to reach a plateau at 60 weeks with the smallest dose of 5 mg/week. With the 10 and 15 mg weekly doses, weight loss continued to the end of trial at 72 weeks but was somewhat attenuated after 60 weeks of therapy. Overview of design and main results of the SURMOUNT-1 trial is summarized in Table 1.
Abbreviations: T5: Tirzepatide 5 mg weekly, T10: Tirzepatide 10 mg weekly, T15: Tirzepatide 15 mg weekly, P: Placebo
Change in Body Composition
The SURMOUNT-1 investigators evaluated change in body fat in a subgroup of 160 participants by dual-energy x-ray absorptiometry (DEXA) [6]. After 72 weeks, percentage reduction in total body fat in the pooled tirzepatide groups was -25.7% (95% CI -31.4 to -20.0) relative to placebo [6]. Moreover, ratio of total fat mass to total lean mass decreased more with tirzepatide (from 0.93 to 0.70) than with placebo (from 0.95 to 0.88) [6].
Effects of Tirzepatide on Cardiovascular Risk Factors
Tirzepatide treatment was associated with significant reductions in mean systolic blood pressure, 7.2 to 8.2 mmHg versus 1.2 mmHg with placebo. Similarly, mean diastolic pressure decreased 4.8 to 5.5 mmHg with tirzepatide versus a mean reduction of 1.0 mmHg with placebo [6]. In addition, tirzepatide was associated with doserelated significant reductions in concentrations of low-densitylipoprotein cholesterol (LDL-C) by 5.3 to 8.6 mg/dl vs 0.9 mg/dl reduction with placebo. Corresponding reductions in triglycerides were 24.3 to 31.4 mg/dl vs 6.3 mg/dl. In addition, mean highdensity lipoprotein cholesterol (HDL-C) concentrations increased by 7.0 to 8.2 mg/dl with tirzepatide vs 0.2 mg/dl with placebo [6]. Tirzepatide lowered fasting glucose levels by 7.7 to 10.6 mg/dl (versus 0.9 mg/dl with placebo) and fasting insulin levels by 42.0 to 49.6 mIU/L (versus 9.7 mIU/L with placebo). Hemoglobin A1c values decreased by 0.4% to 0.5% with tirzepatide versus 0.07% in with placebo. Interestingly, 95.3% of participants in the tirzepatide groups with prediabetes at baseline reverted to normoglycemia at 72 weeks, as compared with 61.9% in the placebo group [6]. All above changes are most likely attributed to the substantial weight loss associated with tirzepatide treatment.
Mechanisms of Anti-Obesity Effects of Tirzepatide
The mechanisms by which terzipatide enhances weight loss are not fully understood and are likely multifactorial. The drug may decrease appetite through actions on central nervous system. In the SURMOUNT-1 trail, 8.6% to 11.5% of tirzepatide-treated patients reported decreased appetite compared with 3.3% in the placebo group [6]. Of note, reduced appetite with tirzepatide was not-dose related [6]. Another factor that may contribute to weight loss induced by tirzepatide is the relatively common occurrence of nausea and vomiting associated with its use (Table 1). Comparison of the extent of weight loss between patients who reported GI adverse effects versus those who did not may clarify this issue. Unfortunately, investigators in the SURMOUNT-1 trial did not report such important information [6].
Safety of tirzepatide
Discontinuation of Tirzepatide Due to Adverse Effects
Overall, tirzepatide was fairly tolerated as reflected by discontinuation rates due to adverse effects which were 4.3-7.1% across tirzepatide groups versus 2.6% with placebo [6].
Gastrointestinal Adverse Effects
The most common adverse effects of tirzepatide are mainly GI. Thus, nausea was reported in 24-33% of patients across tirzepatide groups versus 9% with placebo [6]. Corresponding proportions of diarrhea were 18-23% versus 7%, constipation 11-17% versus 6%, and vomiting 8-12% versus 2%. These GI adverse effects were described as mild to moderate and occurring primarily during the early dose escalation phase of treatment [6]. However, 1-1.9% of participants randomized to tirzepatide discontinued treatment to nausea as compared with 0.3% of individuals in the placebo group. In addition, gall bladder-related events occurred in 1.2 to 2.6% and 0.9% across tirzepatide groups and placebo group, respectively. Importantly, no increase in incidence of pancreatitis was observed although lipase levels increased by 29.2 to 35.3% across tirzepatide groups vs an increase of 5.6% with placebo [6].
Hypoglycemia
Frequency of hypoglycemia (defined as blood glucose < 54 mg/ dl) was 7 to 9 times higher with tirzepatide (1.4-1.6%) compared with placebo (0.2%) [6]. This adverse effect was unexpected because tirzepatide lowered fasting insulin levels as mentioned earlier. In addition, in previous clinical trials of patients with diabetes, tirzepatide did not increase risk of hypoglycemia except when used with insulin or sulfonylureas [4].
Increase Heart Rate
Increase heart was reported with all GLP-1 agonists [7]. Doserelated increase in heart rate of 0.6-2.6 beats per minute (bpm) was reported with tirzepatide versus 0.1 bpm in the placebo group [6]. In addition, 3 tirzepatide-treated participants versus 1 placebotreated subject had severe or serious supraventricular tachycardia and cardiac conduction disorders [6]. Thus, cardiac arrhythmias should be included as one outcome in safety trials of tirzepatide.
Effect of Tirzepatide on Cardiovascular Events and Mortality
In the SURMOUNT-1 trial, no increase in cardiovascular (CV) events was observed with tirzepatide compared with placebo but the number of events was very limited (0.6-0.7% of subjects) due to young age of individuals enrolled and short-duration of study [6]. Similarly, tirzepatide in diabetes trials was not associated with increased CV events. In a meta-analysis of 7 clinical trials with a median duration of follow-up of 55.3 weeks, Sattar et al. [8] have shown that tirzepatide had no significant effects on the 4 major adverse cardiovascular outcomes (MACE-4), namely cardiovascular death, myocardial infarction, stroke and hospitalization for unstable angina.
Appraisal of Tirzepatide as Anti-Obesity Drug
Advantages of Tirzepatide
Semaglutide up to weekly doses of 2.4 mg (Wegovy) was recently approved for treatment of obesity [9]. Although head-tohead comparison between the semaglutide anti-obesity dose of 2.4 mg/week and tirzepatide is not available, indirect evidence suggests that tirzepatide may be superior to semaglutide in the magnitude of weight loss. Thus, placebo-adjusted weight loss with semaglutide (2.4 mg/week) and tirzepatide (15 mg/week) were -12.4% (95% CI -13.3to -11.6) and -17.8% (95% CI, -19.3 to -16.3), respectively [6,10]. In fact, magnitude of weight loss with tirzepatide use may approximate that induced by bariatric surgery. van Rijswijk et al. [11] showed that bariatric surgery results in mean (±SD) weight reduction of 31.9% (8.1) after gastric bypass and 29.5% (9.0) after sleeve gastrectomy at 1 year. In the SURMOUNT-1 trial, 36.2% of participants randomized to tirzepatide 15 mg/week lost ≥ 25% of weight. Therefore, available data suggests that tirzepatide may be the most potent anti-obesity drug, with efficacy approaching that of bariatric surgery after 1 year of use. Other advantages of tirzepatide that occur as result of substantial weight loss include the improvements in CV risk factors such as blood pressure, lipid profile, plasma insulin and glucose, and prevention or delay in onset of type 2 diabetes [6]. Furthermore, tirzepatide was shown to decrease liver fat content (LFC) in patients with diabetes and nonalcoholic fatty liver disease (NAFLD) more than insulin degludec [12]. The estimated treatment difference in LFC versus insulin degludec was -4·71% (95% CI -6·72 to -2·70; p<0·0001) after 52 weeks [12].
Limitations of Tirzepatide
Tirzepatitde has important limitations. First, its efficacy and safety beyond 72 weeks are not known. Second, its use is associated with high incidence of GI adverse effects. Third, unfortunately, the SURMOUNT-1 trial excluded obese individuals with multiple comorbidities. Hence, its results cannot be extrapolated to sicker obese patients in real-life. Fourth, its effects on CV events and mortality require further investigations. The latter issue is of great importance since the drug is likely to be given for long duration, if not life-long. Indeed, weight regain was observed shortly after stopping semaglutide in the STEP 4 trial [13].
Conclusions and Future Directions
Tirzepatide is a promising anti-obesity agent that pertains to the class of dual GIP/GLP-1 agonists. Results of a well-designed 72-week trial suggests that tirzepatide may be the most effective agent for induction of weight reduction, with 17.8% weight loss with the 15 mg/week dose compared with placebo after 72 weeks. Its main adverse effects are GI. Before tirzepatide gets approved for treatment of obesity, its long-term efficacy and safety must be established in further studies. The latter should include obese patients with comorbidities such as diabetes, chronic kidney disease and CV diseases. The impact of tirzepatide on CV events and mortality in patients with diabetes is currently under investigation in the SURPASS-CVOT trial. The latter study is a large (n=12,500) double-blind trial comparing tirzepatide with dulaglutide 1.5 mg/ week and is expected to terminate in October 2024 [5]. Similar trial is needed in obese subjects with different comorbidities.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Fukuda M (2021) The Role of GIP Receptor in the CNS for the Pathogenesis of Obesity. Diabetes 70(9): 1929-1937.
- Coskun T, Sloop KW, Loghin C, Alsina Fernandez J, Urva S, et al. (2018) LY3298176, a novel dual GIP and GLP-1 receptor aganist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol Metab 18: 3-14.
- Willard FS, Douros JD, Gabe MB, Showalter AD, Wainscott DB, et al. (2020) Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight 5(17): 140532.
- Mounjaro (2022) (Tirzepatide). Prescribing information, Lilly USA, LLC, Indianapolis, IN 46285, USA.
- Min T, Bain SC (2021) The Role of Tirzepatide, Dual GIP and GLP-1 Receptor Agonist, in the Management of Type 2 Diabetes: The SURPASS Clinical Trials. Diabetes Ther 12(1): 143-157.
- Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, et al. (2022) Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med.
- Monami M, Nreu B, Scatena A, Giannini S, Andreozzi F, et al. (2017) Glucagon-like peptide-1 receptor agonists and atrial fibrillation: A systematic review and meta-analysis of randomised controlled trials. J Endocrinol Invest 40(11): 1251-1258.
- Sattar N, McGuire DK, Pavo I, Weerakkody GJ, Nishiyama H, et al. (2022) Tirzepatide cardiovascular event risk assessment: A pre-specified meta-analysis. Nat Med 28(3): 591-598.
- Wegovy (2021) (Semaglutide). Prescribing information, Novo Nordisk, Plainsboro, NJ 08536, USA.
- Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, et al. (2021) Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med 384(11): 989-1002.
- Van Rijswijk AS, Van Olst N, Schats W, Van der Peet DL, Van de Laar AW (2021) What Is Weight Loss After Bariatric Surgery Expressed in Percentage Total Weight Loss (%TWL)? A Systematic Review. Obes Surg 31(8): 3833-3847.
- Gastaldelli A, Cusi K, Fernández Landó L, Bray R, Brouwers B, et al. (2022) Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): A substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol 10(6): 393-406.
- Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, et al. (2021) Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults with Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 325(14): 1414-1425.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...