Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5910
Research Article(ISSN: 2638-5910) 
The Effects of Okra (Abelmoschus esculentus (L) Moench) Fruit Extracts on Diabetes Markers in Streptozotocin- Induced Diabetic Rats Volume 3 - Issue 2
Adewale M Esana1*, Kabo Masisib2,3,4, Khuong Leb2, Rotimi E Alukod4, Charles O Olaiyaa1 and Mohammed H Moghadasian2,4
- 1Department of Biochemistry, Faculty of Basic Medical Sciences, University of Ibadan, Ibadan, Oyo State, Nigeria
- 2The Canadian Centre for Agri-Food Research in Health and Medicine, St Boniface Hospital Albrechtsen Research Centre, Canada
- 3Department of Biological Sciences & Biotechnology, Botswana International University of Science and Technology, Botswana
- 4Department of Food and Human Nutritional Sciences, University of Manitoba, Canada
Received:March 12, 2021; Published: March 22, 2021
Corresponding author: Esan Adewale Michael, Department of Biochemistry, Faculty of Basic Medical Sciences, University of Ibadan, Faculty of Basic Medical Sciences, Nigeria
DOI: 10.32474/ADO.2021.03.000160
Abstract
Diabetes is one of the most prevalent metabolic disorders in both developed and developing countries. The use of phytochemicals and dietary agents have gained popularity in the management of this disease. In this study, aqueous and ethanol extracts from okra fruits produced by conventional plants or plants pre-treated with Indole Acetic Acid (IAA) were tested for anti-diabetic efficacy. These effects were compared with those of glibenclamide in a Streptozotocin (STZ)-induced diabetic rat model over 6 weeks. Injection of STZ resulted in a full-blown phenotype of diabetes. By 4 weeks of the study, plasma glucose, total cholesterol and triglycerides levels were significantly increased when compared with those in non-diabetic control rats. These changes were associated with significant decreases in body weight and increases in food consumption. Daily administration of okra extracts or glibenclamide did not generate a significant effect on body weight status, food consumption or glucose levels. However, aqueous extracts from IAAokra resulted in significant reductions in plasma cholesterol and triglycerides levels. These lipid-lowering effects of IAA-extracts were similar to those of glibenclamide in this animal model. Future studies warrant an investigation of lipid-lowering agents of aqueous extracts of IAA-okra fruits in this and other animal models.
Keywords: Abelmoschus Esculentus; Indole Acetic Acid; Aqueous Extracts; Ethanol Extracts; Diabetic Rats; Streptozotocin
Introduction
Diabetes Mellitus (DM) is a metabolic disorder characterized by hyperglycaemia and alterations in glucose metabolism [1]. Based on pathogenesis, there are two types of diabetes, namely type 1 and type 2; prevalence, distribution of the disorder and populations at risk as well as the management vary between the two types [2]. Type 1 is developed because of a lack of insulin; therefore, insulin therapy is a must for these subjects. Patients with type 2 are generally adults and generally have insulin resistance as an underlying cause of the disease. Appropriate diets and lifestyle can play a crucial role in the management of type 2 diabetes with respect to individuals who may not make enough insulin and/ or develop insulin resistance [3]. Subjects with type 2 diabetes also develop other metabolic disorders, including cardiovascular, kidney, and nerve diseases [4]. Obesity and lipid disorders are also common among subjects with type 2 diabetes, making them at a high risk for cardiovascular diseases.
Depending on the severity of hyperglycemia and diabetes complications, pharmacological agents may be used in the management of diabetes. However, pharmacological agents usually elicit one or more side effects, which may not be tolerated by a number of patients [5]. On the other hand, ethnomedicine (alternative therapy) has gained popularity among these subjects [6]. Many bioactive compounds with antidiabetic activity from various medicinal plants have been isolated and studied, but scientists are still searching for medicinal plants with strong antidiabetic effect with little or no side-effects. Many plant metabolites, like alkaloids, flavonoids, and phenolic acids have been shown to possess health-promoting properties [7]. Various approaches have been developed to increase the production of such functional substances in agricultural products. One of such approaches is the utilization of Indole Acetic Acid (IAA) in agricultural research. Indole acetic acid is involved in virtually all developmental processes in plants, and as such, it is one of the major endogenous regulators of fruit development and metabolism [8].
Okra (Abelmoschus esculentus (L) Moench) is a tropical vegetable that has been used extensively in traditional medicine for DM treatment. The fruit is very rich in minerals, and a good source of antioxidant vitamins, with very low fat and calories contents [9]. The fruits are widely used for medicinal purposes with considerable industrial values. The fibre in okra helps to normalise blood sugar by regulating the rate at which sugar is absorbed from the intestinal tract [10]. The plant is also known for its potential to manage other diseases and disorders [11]. Also, polysaccharides in okra reduce plasma cholesterol levels and may prevent some cancers by its ability to bind bile acids [12]. Previous studies have confirmed that A. esculentus peel and seed possess blood glucose normalization and lipid profiles lowering action in diabetic condition [13,14]. There are limited or no study on antidiabetic activity of indole acetic acid-treated okra fruit powder extract in diabetic rats. Therefore, we hypothesized that okra fruit generated by IAA-treated okra plants have superior antidiabetic effects when compared with the effects of conventional okra fruits. Therefore, this study was conducted in order to investigate the antidiabetic activity of water and ethanol-extracts of conventional and IAA-treated okra fruit powder in streptozotocin (STZ)-induced diabetic rats.
Materials and Methods
Ethical clearance
Ethical approval was obtained from the Animal Ethics Committee of University of Manitoba under project number 13- 053/2 and followed the University of Manitoba Guides for the Care and Use of Laboratory Animals.
Collection of Plant Material
Based on our previous study [15], okra fruits from the indole acetic acid-treated (IAA-treated) and non-treated (control) okra plants were used.
Plant Extracts Preparation
The okra fruits were washed and air-dried at room temperature for a duration of 6-week. The dried fruits were pulverized using mortar and pestle and sieved through sieve no. 40; the produced okra powder was stored in airtight containers at -20°C. The okra powder samples (250 g) were subjected to extraction procedures using 10 volumes of either 75% ethanol or distilled water on a magnetic stirrer at 40°C for 3 h. The ethanol-extract was decanted, filtered and transferred into the rotary evaporator equipment for total removal of ethanol under vacuum; the aqueous residue was freeze-dried. The aqueous extract was stirred until a viscous mixture was obtained, which was then boiled for 10 min, and allowed to cool followed by centrifugation for 10 min at 12,500g; the supernatant was then freeze-dried. The freeze-dried powders from ethanol and aqueous extractions were stored at 4°C during the entire course of the study.
Animals
Twenty-eight male Sprague-Dawley rats weighing 130 ±15 g of four weeks of age were used in 7 groups of 4 rats each (n=4 for each group) for this study as listed below. The rats were housed in groups of 2 in conventional cages in a room with controlled temperature (24°C ± 1°C) and a 12:12-hour light-dark cycle with ad libitum feeding.
Induction of Diabetes
The rats were injected with 65 mg/kg bw STZ (prepared in saline solution) through the tail vein. Blood glucose levels after one week were estimated using glucometer and animals with a glucose level of 250 mg/dL or higher were used in the feeding study. Control animals were injected with saline.
The Study had the Following Experimental Groups
Group A: Non-diabetic control animals (no treatment)
Group B: Control diabetic rats (diabetic no treatment)
Group C: Diabetic rats received aqueous-extract of control okra at 100 mg/kg BW.
Group D: Diabetic rats received aqueous extract of IAA-treated okra at 100 mg/kg BW.
Group E: Diabetic rats received ethanol-extract of control okra at 100 mg/kg BW.
Group F: Diabetic rats received ethanol extract of IAA-treated okra at 100 mg/kg BW
Group G: Diabetic rats received 5 mg/kg bw of glibenclamide. Okra fruit extracts and the drug were administered daily to all the animals orally (gavage) for the period of six weeks. Body weight, blood glucose levels and food intake were measured weekly. Blood samples were collected from the Jugular vein bi-weekly under light anaesthesia for total cholesterol and triglyceride measurements. After six weeks of treatment, the rats were euthanized using carbon dioxide followed by cardiac puncture; the final blood samples were collected for biochemical analysis using pre-heparinised syringes. Various internal tissues including the heart, aorta, spleen, abdominal fat, liver, and kidneys were also collected, weighed, and stored at −80°C until analysis. Tissue specimens were fixed in 10% buffered formalin and sectioned for histologic examinations.
Determination of Blood Glucose
Whole blood glucose concentrations were measured on blood samples taken from tail vein using a glucometer and strips (AlphaTRAK2 glucometer) as previously described by Owiredu et al, [16].
Plasma Lipids
Plasma levels of Total Cholesterol (TC) and Triglycerides (TG) were measured in plasma samples taken bi-weekly from fasting animals per standard enzymatic procedures [17].
Plasma Superoxide Dismutase (SOD) Activities
Randox kits (Randox Laboratories) were used to estimate superoxide dismutase activities on plasma samples. Superoxide dismutase activities determination was based on the production of superoxide (O2-) anions by the xanthine oxidase system [18]. Superoxide reacts with I.N.T (2-(4-iodophenyl)-3-(4-nitrophenol)- 5-phenyltetrazolium chloride) to form a red formazan dye. The activity of SOD was estimated by the degree of inhibition of this reaction, which was measured at 505 nm using UV/VIS spectrophotometer. The results were expressed as units per milligram of protein.
Plasma and Liver Total Phenolic Contents
Preparation of Liver for the Folin–Ciocalteu Assay: The liver specimens were placed into 50 mL centrifuge tubes and homogenised with 10 mL of distilled water. Homogenisation, sonication, centrifugation and filtration steps to obtain an extract were performed as described by Luciano et al, [19]. A 1: 4 dilution of the extract (3 mL of distilled water added to 1 mL of liver extract) was chosen. Briefly, 100 μL of the diluted liver extract was transferred into 15 mL centrifuge tubes and 900 μL of distilled water were added. The Folin–Ciocalteu reagent was diluted to 1 N and 500 μL were added to the tubes followed by 2.5 mL aqueous solution of sodium carbonate (20%; w/v). The mixture was vortexed mixed for 30 s and incubated for 40 min in the dark at room temperature. The samples were centrifuged at 2700×g for 10 min at 4°C to remove any sodium carbonate precipitates. A doublebeam spectrophotometer (model UV-1601, Shimadzu Corporation, Milan, Italy) was used to measure the absorbance of the samples at 725 nm. A vial containing all reagents, except the tissue extract, was used as blank. Aqueous solutions of garlic acid were used for the calibration curve, which covered 0 to 80 μg/μL of garlic acid. The results were expressed as mg of garlic-acid equivalents (GAE)/g of liver. For the plasma samples, 100 μL of the plasma were transferred to 15 mL centrifuge tubes and diluted with 900 μL of distilled water. The Folin–Ciocalteu assay was carried out as described for the liver samples. The results were expressed as mg of GAE/mL of plasma.
Histological and Immunohistological Examinations: The liver specimens were processed and sectioned for light microscopy examination after application of Periodic Acid Schiff (PAS) and hematoxylin eosin (H&E) staining per our previously reported procedures. Pancreatic tissues were also processed and stained with H&E as well as primary and secondary antibodies against insulin for determination of the density and distribution of betacells [20].
Statistical Analysis
ANOVA 2-way analysis followed by Bonferroni’s Multiple Comparison Test was used to perform statistical analysis on data from all experimental groups. Data are presented as means and standard deviations. P<0.05 was considered to identify statistically significant differences among the groups.
Results
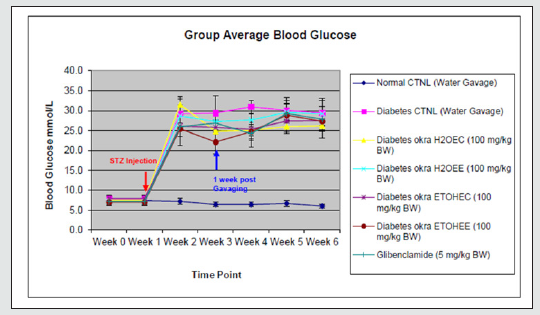
Body Weight and Food Intake
Changes in body weight, tissue weight and the level of food intake are presented in Table 1. A steady body weight gain was observed in all animals until 2 weeks after STZ injection. Induction of diabetes state by STZ injection resulted in a significant reduction in weight gain rates. By the end of the studies at week 6, none of the treatment protocol resulted in maintaining normal body weight gain. Thus, at the end of the experiments all of diabetic rats had significantly (p<0.05) lower body weight when compared with the body weight of non-diabetic control animals. The induction of diabetes was associated with increased food intake in all of the animals. As shown in Table 1, on average the diabetic animals consumed approximately 3 times more food as compared to the non-diabetic control animals. This level of increased food intake remained constant during the experimental course.
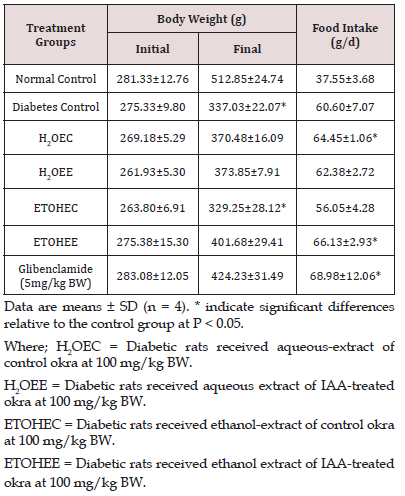
Blood Glucose Level
Figure 1 shows blood glucose levels in all groups of experimental rats. Injection of STZ resulted in a diabetic state within 2 weeks and stayed constant during the 4 weeks of the remaining experimental course. Blood glucose levels increased by almost 3 times in all experimental groups. None of the treatment protocol resulted in significant reductions in blood glucose levels in diabetic animals.
Where; Normal CTNL = Normal control
Diabetes CTNL = Diabetes control
H2OEC = Diabetic rats received aqueous-extract of control okra at 100 mg/kg BW.
H2OEE = Diabetic rats received aqueous extract of IAA-treated okra at 100 mg/kg BW.
ETOHEC = Diabetic rats received ethanol-extract of control okra at 100 mg/kg BW.
ETOHEE = Diabetic rats received ethanol extract of IAA-treated okra at 100 mg/kg BW.
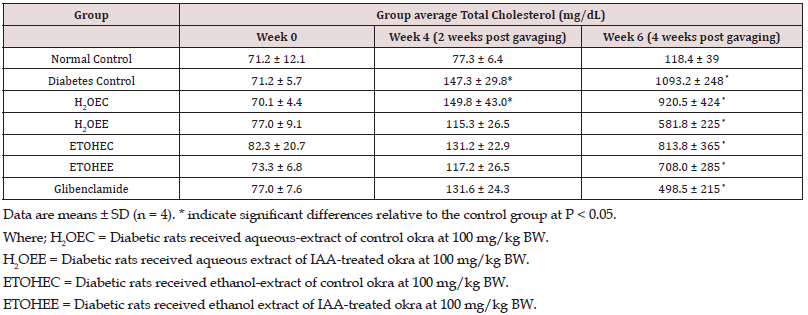
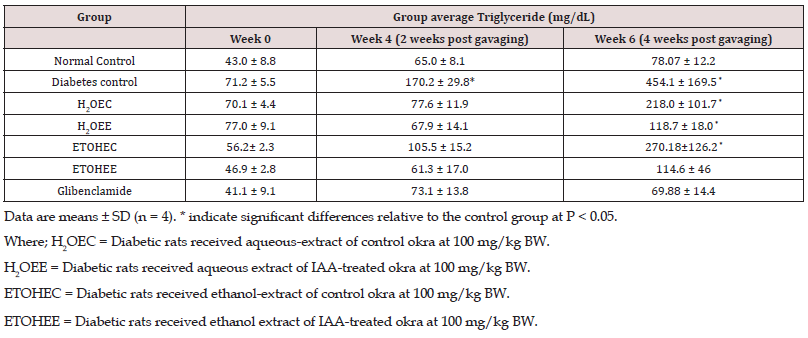
TG and TC Levels Determination in Serum
In Table 2, serum Total Cholesterol (TC) levels were comparable among all experimental groups by week 4 of the study. However, these levels increased in all diabetic rats at week 6 of the study by different extents. The increments in plasma total cholesterol levels by the end of the experiments were as follow: non-diabetic control rats by 66%; non-treated diabetic control animals by approximately 1400% (p<0.05); control okra water extract-treated diabetic rats by 1214% (p<0.05); IAA-okra water extract-treated diabetic rats by 654%; control okra ethanol extract-treated diabetic rats by 891% (p<0.05); IAA-okra ethanol extract-treated diabetic rats by 870%; and glibenclamide-treated diabetic rats by 546%. Similar to plasma cholesterol levels, plasma triglycerides levels were steady and comparable among all experimental rats by week 4 of the study. However, during the last 2 weeks of the experimental course the levels of triglycerides were increased by a different degree in experimental rats. These increases include: non-diabetic control rats by 81%; non-treated diabetic control animals by approximately 540% (p<0.05); control okra aqueous extract-treated diabetic rats by 211% (p<0.05); IAA-okra aqueous extract-treated diabetic rats by 53%; control okra ethanol extract-treated diabetic rats by 382% (p<0.05); IAA-okra ethanol extract-treated diabetic rats by 142%; and glibenclamid-treated diabetic rats by 70% (Table 3).
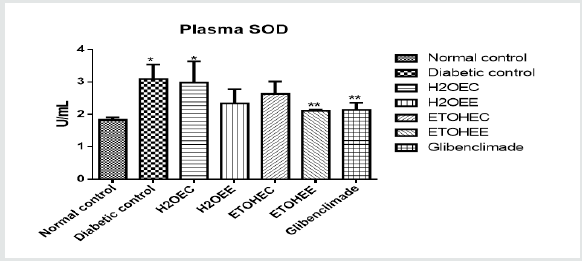
Plasma SOD Activities
Data: Means ± SD (n = 4)
*Significant at P < 0.05 relatives to the control group.
**Significant at P < 0.05 relatives to the experimental groups.
Where; H2OEC = Diabetic rats received aqueous-extract of control okra at 100 mg/kg BW.
H2OEE = Diabetic rats received aqueous extract of IAA-treated okra at 100 mg/kg BW.
ETOHEC = Diabetic rats received ethanol-extract of control okra at 100 mg/kg BW.
ETOHEE = Diabetic rats received ethanol extract of IAA-treated okra at 100 mg/kg BW.
Glibenclamide at 5 mg/kg BW.
Figure 2 shows plasma SOD activities in all experimental rats. Compared with non-diabetic control rats, only 2 groups of non-treated diabetic rats and control okra water extract-treated rats showed significantly (p<0.05) higher levels of SOD activities. All other treated-diabetic rats showed comparable levels of SOD activities.
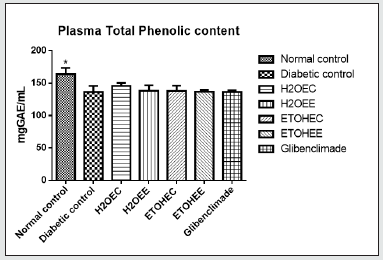
Plasma and Liver Total Phenolic Concentrations
Data: Means ± SD (n = 4)
*Significant at P < 0.05 relatives to the experimental groups.
Where; H2OEC = Diabetic rats received aqueous-extract of control okra at 100 mg/kg BW.
H2OEE = Diabetic rats received aqueous extract of IAA-treated okra at 100 mg/kg BW.
ETOHEC = Diabetic rats received ethanol-extract of control okra at 100 mg/kg BW.
ETOHEE = Diabetic rats received ethanol extract of IAA-treated okra at 100 mg/kg BW.
Glibenclamide at 5 mg/kg BW.
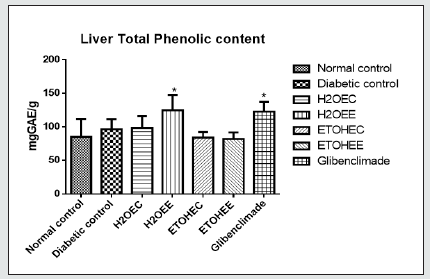
Data: Means ± SD (n = 4)
*Significant at P < 0.05 relatives to the control group.
Where; H2OEC = Diabetic rats received aqueous-extract of control okra at 100 mg/kg BW.
H2OEE = Diabetic rats received aqueous extract of IAA-treated okra at 100 mg/kg BW.
ETOHEC = Diabetic rats received ethanol-extract of control okra at 100 mg/kg BW.
ETOHEE = Diabetic rats received ethanol extract of IAA-treated okra at 100 mg/kg BW.
Glibenclamide at 5 mg/kg BW.
Total phenolic concentrations in the plasma and liver tissue have been presented on (Figures 3&4). All 6 groups of diabetic rats had significantly (p<0.05) lower total phenolic concentrations in their plasma when compared with those in the non-diabetic control rats (Figure 3). However, the levels of phenolic compounds in the liver tissues were comparable among all groups of rats (Figure 4).
Liver and Pancreas Histology
Examinations of sections from the liver and pancreatic tissues from the experimental animals did not reveal major differences among the group, except for decreased insulin reactivity in the pancreas tissues of the STZ-injected animals.
Discussion
Diabetes mellitus has been characterized by hyperglycaemia, insulin resistance, and relative insulin deficiency [21]. The use of phytochemicals with antioxidant and other beneficial properties to protect pancreatic β-cells would be of great clinical relevance. On this note, the present study was designed to test the influence of Abelmoschus esculentus extracts in diabetic Sprague-Dawley rats. STZ induces diabetes by disrupting pancreatic β-cells [22]. In the present study, after the administration of STZ, a significant increase in blood glucose levels was observed after one week. The hyperglycaemic conditions were also associated with a progressive body weight loss and a significant increase in food intake in the experimental rats. These changes were further accompanied by significant increases in plasma total cholesterol and triglycerides levels, which took two weeks after STZ injection to develop and continued in a stable matter until the end of the experiments. Thus, injection of STZ was effective to induce a full-blown state of diabetes and all of its complications, including lipid disorders, body weight loss and increased food intake within a 4-week induction course. In tandem with this study, Waer [23] observed a necrotic change in pancreatic β-cells islets in diabetic rats induced with STZ. Also, shrinkage and condensation of the nuclear materials in pancreatic β-cell reported by Arulselvan and Subramanian [24] supported the findings of this present study.
.Treatment of these animals with aqueous or ethanol extracts of either conventional okra or okra produced by plants pre-treated with IAA resulted in interesting findings. We compared the effects of okra extracts with those of a well-studied pharmacological agent, namely glibenclamide over 6 weeks of the experimental course. Treatment of diabetic rats with okra extracts did not result in prevention of weight loss in any group of the rats. Interestingly, the effects of okra extracts in this regard were similar to those of gilbenclamide. Similarly, none of the treatment protocols could normalize food consumption in any of the experimental groups. Unlike our study, the study of Sezik et al, [25] reported increased body weight in the diabetic animals after the Administration of Flavonoid Biochanin A (BCA). The reason for this disagreement could be due to the types and amounts of flavonoids present in okra extracts. Indeed, our data show a decrease in total phenolic compounds in the plasma of all diabetic animals when compared with those in non-diabetic control rats. This indicates that okra extracts regardless of the type of okra fruits or the method of extractions were not able to cause an increase in either plasma or hepatic contents of phenolic compounds.
A lack of increases in plasma and hepatic phenolic compounds could also explain the lack of glucose-lowering properties of the okra extracts. A similar speculation can be reached for the comparable SOD activities observed in diabetic animals. Kaleem et al, [26] also observed a decrease in SOD and CAT activity in the diabetic rats; these authors proposed that it might be due to over-production of reactive oxygen species in diabetic animals. SOD is involved in the detoxification of superoxide anion, by converting it into hydrogen peroxide and water, while catalase catalyses the reduction of H2O2 to water and oxygen, and thus prevent harmful effects of hydroxyl radicals in the tissues [27]. The decreased activity of antioxidant enzymes observed in the diabetic rats, may be due to glycation of the enzyme in response to hyperglycaemia. Previous studies have reported both glucose-lowering and antioxidant activities for phenolic compounds [28]. A lack of glucose-lowering effects of okra extracts could be explained by comparable density and distribution of insulin-producing cells observed in the pancreatic tissues of these animals. It is interesting that the anti-obesity efficacy of okra extracts was similar to that of glibenclamide in this study. Both types of okra extracts were able to reduce elevated levels of cholesterol and triglycerides in diabetic animals. Particularly, aqueous extract of IAA-okra was the most effective agent for reducing the elevated levels of total cholesterol and triglycerides. It is interesting that these effects of aqueous extracts of IAA-okra products are comparable with those of glibenclamide. At this time, it is not clear what was the functional ingredients in the aqueous extracts of IAA-okra products. Future studies should focus on functional properties of this product in this and other animal models of metabolic disorder.
Conclusion
In conclusion, we report that STZ-induced diabetes in rats is associated with significant abnormalities in weight gain, food intake, glucose and lipid metabolism. Neither glibenclamide nor okra extracts were able to normalize glucose metabolism and the abnormalities in body weight and food intake. This lack of efficacy could be related to inadequate doses or bioavailability of the functional ingredients as no changes were observed in total phenolic contents of plasma and liver tissues. Another explanation could be the degree of severity of diabetes. It can be suggested to test these extracts in animal models of type 2 diabetes. The most interesting finding of this study relates to the efficacy of aqueous extracts of IAA-okra on hyperlipidaemia and its similarity to glibenclamide in this animal model.
Funding
This research was supported by the Tertiary Education Trust Fund (TET Fund) for Post Graduate Grant.
Acknowledgments
We are grateful to the R.O. Burrel laboratory staff, St Boniface Hospital Albrechtsen Research Centre, and to the research activities of Mohammed H. Moghadasian which is supported by an Individual Discovery from Natural Sciences and Engineering Research Council of Canada.
References
- Georg P, Ludvik B (2000) Lipids and Diabetes. J Clin Basic Cardiol 3(3): 159-162.
- Ashok kumar N, Pari L (2005) Effect of N-Benzoyl-D-Phenylalanine and metformin on carbohydrate metabolic enzymes in neonatal streptozotocin diabetic rats. Clin Chim Acta 351(1-2): 105-113.
- Chen J, Li WL, Wu JL, Ren BR, Zhang HQ (2008) Hypoglycemic effects of a sesquiterpene glycoside isolated from leaves of loquat (Eriobotrya japonica (Thunb.)Lindl.) Phytomed 15(1-2): 98-102.
- Yang K, Jeong SC, Lee HJ, Sohn DH, Song CH (2006) Antidiabetic and hypolipidemic effects of Collybia confluens mycelia produced by submerged culture in streptozotocin diabetic rats. Arch Pharm Res 29(1): 73-79.
- Palsamy P, Subramanian S (2008) Resveratrol, a natural phytoalexin, normalizes Hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed Pharmacother 62(9): 598-605.
- Chandramohan G, Ignacimuthu S, Pugalendi KV (2008) A novel compound from Casearia esculenta (Roxb.) root and its effect on carbohydrate metabolism in streptozotocin-diabetic rats. Eur J Pharmacol 590(1-3): 437-443.
- Wang Y, Luo Z, Lu C, Zhou R, Zhang H, et al. (2019) Transcriptome profiles reveal new regulatory factors of anthocyanin accumulation in a novel purple-colored cherry tomato cultivar Jinling Moyu. Plant Growth Regul 87: 9-18.
- De Jong M, Wolters-Arts M, Feron R, Mariani C, Vriezen WH (2009) The Solanumly copersicum auxin response factor 7 (SIARF7) regulates auxin signalling during tomato fruit set and development. Plant J 57(1): 160-170.
- IBPGR (International Board for Plant Genetic Resources) (1990) Report on International Workshop on okra Genetic Resources held at the National Bureau for plant Genetic Resources. New Delhi, India, 8-12.
- Sengkhamparn N, Verhoef R, Schols HA, Sajjaanantakul T, Voragen AG (2009) Characterisation of cell wall polysaccharides from okra (Abelmoschus esculentus (L.)Moench). Carbohydr Res 344(1): 1824-1832.
- Sabitha V, Ramachandran S, Naveen KR, Panneerselvam K (2013) Anti-diabetic and antihyperlipidemic potential of Abelmoschus esculentus (L.) Moench. In streptozotocin-induced diabetic rats. J of Pharm and Bioallied Sci 3(3): 397-402.
- Kahlon TS, Chapman MH, Smith GE (2007) In vitro binding of bile acids by okra, beets, asparagus, eggplant, turnips, green beans, carrot and cauliflower. Food Chem 103(2): 676-680.
- Sabitha V, Ramachandran S, Naveen KR, Panneerselvam K (2012) Investigation of in vivo antioxidant property of Abelmoschus esculentus (L) moench. fruit seed and peel powders in streptozotocin-induced diabetic rats. J of Ayurv and Integrat Med 3(4): 188-193.
- Ramachandran S, Sandeep VS, Srinivas NK, Dhanaraju MD (2010) Anti-diabetic activity of Abelmoschus esculentus Linn. on alloxan-induced diabetic rats. Res and Rev in Bio Sci 4(3):121-123.
- Esan AM, Masisi K, Dada FA, Olaiya CO (2017) Comparative effects of indole acetic acid and salicylic acid on oxidative stress marker and antioxidant potential of okra (Abelmoschus esculentus) fruit under salinity stress. Sci Horticult 216: 278-283.
- Owiredu WKBA, Amegatcher G, Amidu N (2009) Precision and accuracy of three blood glucose meters: Accu-Check advantage, One Touch horizon and Sensocard. J Med Sci 9(4): 185-193.
- Moghadasian MH (2006) Dietary phytosterols reduce cyclosporine induced hypercholesterolemia in apo E-KO Mice. Transplantation 81(2): 207-213.
- Nurdiana S, Goh YM, Ahmad H, Dom SM, Syimalain AN, et al. (2017) Changes in pancreatic histology, insulin secretion and oxidative status in diabetic rats following treatment with Ficus deltoidea and vitexin. BMC Comp and Alter Med 17(1): 1-17.
- Luciano G, Vasta V, Monahan FJ, López-Andrés P, Biondi L, et al. (2011)Antioxidant status, colour stability and myoglobin resistance to oxidation of longissimus dorsi muscle from lambs fed a tannin-containing diet. Food Chem 124(3): 1036-1042.
- Fairbanks V, Klee G (1996) Biochemistry aspects of haematology. Tietz Fundamental of Clinical Chemistry (4th edn); Philadelphia, WB Saunders Co. 704-730.
- Olokoba AB, Obateru OA, Olokoba LB (2012) Type 2 Diabetes Mellitus: A Review of Current Trends. Oman Med J 27(4): 269-273.
- Fu Z, Zhang W, Zhen W, Lum H, Nadler J, et al. (2010) Genistein induces pancreatic β-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology 151(7): 3026-3037.
- Waer H F (2012) Cytological and histochemical studies in rat liver and pancreas during progression of streptozotocin induced diabetes and possible protection of certain natural antioxidants. J Nutri & Food Sci 2(9): 165.
- Arulselvan P, Subramanian SP (2007) Beneficial effects of Murraya koenigii leaves on antioxidant defense system and ultrastructural changes of pancreatic β-cells in experimental diabetes in rats. Chemico-Biological Interactions 165(2): 155-164.
- Sezik E, Aslan M, Yesilada E, Ito S (2005) Hypoglycaemic activity of Gentiana olivieri and isolation of the active constituent through bioassay-directed fractionation techniques. Life Sci 76(11): 1223-1238.
- Kaleem M, Asif M, Ahmed QU, Bano B (2006) Antidiabetic and antioxidant activity of Annona squamosal extract in streptozotocin-induced diabetic rats. Singapore Med J 47(8): 670-675.
- Searle AJ, Wilson RL (1980) Glutathione peroxidase: Effect of superoxide, hydroxyl and bromine free radicals on enzyme activity. Int J Radiat Biol Relat Stud Phys Chem Med 37(2): 213-217.
- Kumar P, Kale RK, Mukherjee S, Prakash K, McLean P, et al. (2011) Antidiabetic effects of Trigonella foenum-graecum seed powder in a rat model. Toxicol Environ Chem 93(10): 2085-2097.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...