Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4692
Research Article(ISSN: 2637-4692) 
The contribution of fatigue on the transition from acute to chronic painful temporomandibular disorders, and its persistence: a prospective 3-month cohort study Volume 5 - Issue 4
Sherif M Elsaraj1,2, Gornitsky Mervyn1,2, Richard Hovey1, Firoozeh Samim1, Zovinar Der Khatchadourian1 and Ana Velly1,2*
- 1Faculty of Dentistry, McGill University, Canada
- 2Department of Dentistry, Jewish General Hospital, Canada
Received: November 10, 2022; Published: November 23, 2022
Corresponding author: Ana Miriam Velly, Associate Professor, McGill University, Faculty of Dentistry Department of Dentistry, Jewish General Hospital 3755 Cote St-Catherine, Suite A.017 Montreal, Quebec, H3T 1E2, Canada
DOI: 10.32474/MADOHC.2022.05.000219
Abstract
Previous studies have demonstrated that fatigue is associated with chronic temporomandibular disorders (TMD) pain. TMD is a group of a musculoskeletal condition affecting the muscles of mastication, the temporomandibular joints, or both. This prospective cohort study determines whether fatigue is associated with the transition from acute to chronic TMD-related pain as well as its’ persistence, when chronic pain is defined by: (i) duration ( > 3 months), and (ii) dysfunction (Graded Chronic Pain Scale (GCPS IIIV). The International Association for the Study of Pain (IASP) defines chronic pain as “persistent or recurrent pain lasting longer than 3-months” and is associated with significant dysfunction. From 454 subjects recruited between 2015 to 2021, through four locations in Canada, 376 completed the follow-up. A diagnosis was obtained using the Research Diagnostic Criteria or the Diagnostic Criteria for Temporomandibular Disorders. Fatigue was assessed at baseline with the Fatigue Severity Scale. Subjects completed the GCPS form at baseline and 3-month follow-up. When chronic pain was defined as dysfunction, fatigue was associated with an increased transition or persistence risk (RR adjusted = 1.72, P = 0.002), contrary to pain duration (RR adjusted =1.01, P = 0.99). This multivariable analysis was adjusted by baseline acute and chronic pain status, dysfunction, and sex. Results indicate that fatigue contributed to the transition and persistence of chronic TMD-related pain risk at a 3-month follow- up when chronic pain was defined by dysfunction contrary to defined by duration. This result suggests that fatigue assessment may be an essential part of a comprehensive clinical examination.
Keywords: Temporomandibular disorders; chronic pain; acute pain; transition; cohort study; risk factors; fatigue; disability; dysfunction
Introduction
Temporomandibular disorders (TMD) is a collective term used to describe musculoskeletal conditions characterized by pain in the muscles of mastication and temporomandibular joint or both, and/or associated structures [1]. The common signs and symptoms include tenderness in the muscles upon palpation, pain within the range of motion, or limitation of the jaw upon opening. This is followed by interference with vital functions such as eat ing, swallowing, and speaking [20]. The prevalence of TMD-related pain ranges between 5% to 12% [10,23] and the annual incidence is 3.9% [16]. TMD-related pain is more common among females than males [23]. Nevertheless, approximately 33% of TMD-related pain patients continue to suffer from moderate to severe levels of pain and disability, independent of treatment received [13,14]. Fatigue is a subjective experience with symptoms including a persisting lack of energy, exhaustion, physical and mental tiredness, and apathy [2]. The prevalence of fatigue varies from 0.2% to 6.41%[11] Fatigue prevalence is considerably higher among subjects with TMD-related pain (14-43%) [2,5,9]. Dahan et al. [5] found that fatigue was positively associated with chronic TMD-related pain intensity and duration. The current prospective cohort study is part of the Acute to Chronic TMD Transition (ACTION) program, with an overall goal to identify the risk factors implicated in the transition from acute to chronic TMD-related pain and its persistence.
The current study aimed to assess whether fatigue was associated with the transition from acute to chronic TMD-related pain risk as well as with its persistence at a 3-month follow-up. Thus, the specific aims are:
a) Aim 1: To determine if fatigue is associated with the transition and persistence risk when chronic TMD-related pain is defined as recurrent or persistent pain for more than 3 months.
b) Aim 2: To determine the contribution of fatigue on the transition or persistence risk when chronic TMD-related pain is defined by dysfunction as classified by GCPS (Graded Chronic Pain Scale Grades II-IV) [24].
The rationale to define chronic pain based on pain duration and dysfunction is described below. First, the International Association for the Study of Pain (IASP) defines chronic pain as recurrent or persistent pain lasting for more than 3 months [18,19]. Second, IASP states that chronic pain is associated with significant disability [19]. Therefore, chronic TMD-related pain was also defined as a dysfunction state consisting of clinically significant pain and disability [24]. The study hypotheses are that fatigue increases the risk of a transition from acute to chronic TMD- related pain as well as its persistence when chronic TMD-related pain is defined by pain duration or dysfunction. To date, we are not aware of any study investigating the contribution of fatigue on the transition from acute to chronic TMD-related pain as well as its persistence.
Methods
Study design and study population
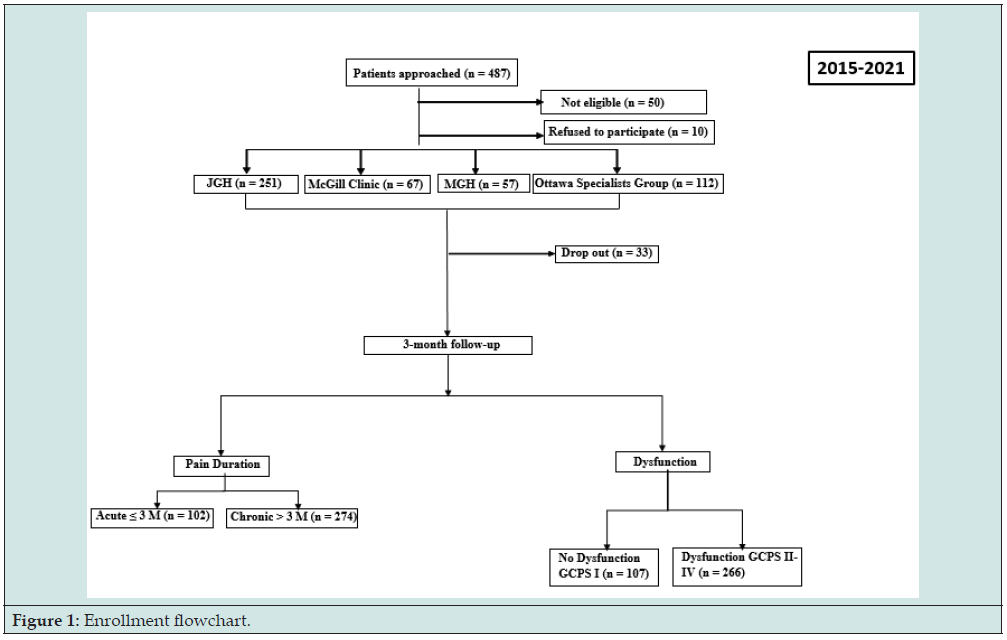
The Acute to Chronic TMD Transition (ACTION) program received approval from the McGill Institutional Review Board in Montreal, Canada (approval number: A12-M113-14A) and by the Dental Specialists Group in Ottawa, Ontario (approval number: 240-400). Eligible subjects with acute or chronic TMD-related pain were recruited between August 2015 and March 2021 from four different sites: the Jewish General Hospital (JGH) general dental clinic, the Faculty of Dentistry of McGill University oral diagnosis (OD) clinic, the Montreal General Hospital dental department (MGH), and the Dental Specialists Group TMD-specialized clinic (Figure 1). The inclusion criteria for the participation in this study required subjects to be of 18 to 85 years of age with a positive diagnosis of TMD-related pain (muscle and/or joint) in accordance with Research Diagnostic DC [6] or the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) [15]. The excluded subjects were those who had other orofacial pain (e.g., dental pain), cancer, no access to a telephone, those who were unable to provide informed consent or incapable of understanding French or English.
Acute and chronic pain classification
We used two criteria to define acute and chronic TMD pain: (i) pain duration of chronic TMD-related pain is in accordance with the International Association for the Study of Pain (IASP) which defines chronic pain as pain lasting for more than 3 months [18, 19], and (ii) dysfunction defined as grades II, III and IV with any disability points on the Graded Chronic Pain Scale (GCPS) [24].
GCPS is an instrument used to assess overall chronic pain severity based on the level of pain intensity and pain-related disability. The GCPS grades are low-intensity pain, no disability (Grade I); high-intensity pain, without pain-related disability (Grade IIa); (iii) high-intensity pain, with low pain-related disability (Grade IIb), moderately limiting (Grade III), and severely limiting (Grade IV). The scoring is based on the subject’s responses to several items: 1) current; 2) worst; and 3) average pain intensity (0–10 numeric scales); 4) pain-related disability days, and pain-related interference with daily activities; work; and social or family activities (0– 10 numeric). Characteristic pain intensity (CPI) measured by the GCPS is the average of 0–10 ratings of current, worst and average pain in the prior 3 months multiplied by 10. The disability score is the average of 3, 0–10 interference ratings: daily activities, work, and social or family activities multiplied by 10 prior 3 months.
Outcome variables
The primary outcomes were the transition from acute to chronic TMD-related pain or the persistence of chronic pain at the 3-month follow-up when chronic pain was defined by pain duration and dysfunction. Secondary outcomes were the transition and the persistence state, both also defined by pain duration and dysfunction.
Assessment of fatigue
Fatigue severity and functionality were assessed using the Fatigue Severity Scale (FSS), a validated and reliable instrument [21]. The FSS has 90% sensitivity and 86% specificity, with high reliability (Cronbach α = 0.93). The scoring cutoffs are: < 35: no fatigue; and ≥ 36: fatigue.
Putative confounders and effect modifiers assessment
In our study, the possible confounders all assessed at baseline were acute and chronic pain status, dysfunction, psychological factors (anxiety, depression), CPI, sex, and age. The Patient Health Questionnaire-4 (PHQ-4) is a validated and reliable instrument used for screening for psychological factors: anxiety and depression [12]. PHQ-4 scoring cutoffs are: < 3: no anxiety and depression; and ≥ 3: anxiety and depression.
Statistical analysis
Chi-squared, Fisher’s exact test, analysis of variance, and Student t-test were used to test statistical differences between categories of TMD-related pain groups relative to fatigue, acute and chronic pain status, GCPS grades (GCPS I-IV), CPI, age, sex, and psychological factors.
Primary analysis
For aim 1, we conducted binary logistic regression. The dependent variable was the presence or absence (yes or no) of chronic TMD-related pain at 3-month of follow-up. The risk factor under study was fatigue (yes or no) and the putative confounders were acute-chronic pain status at baseline, age, sex, CPI, and psychological factors. For aim 2, a binary logistic regression analysis was also performed to assess the relative risk of fatigue. The dependent variable was GCPS at 3-month of follow-up: 0-I (no dysfunction) vs and II-IV (dysfunction). The risk factor was fatigue (yes or no) or fatigue score (yes or no) and the putative baseline confounders dysfunction were acute-chronic pain status at baseline, age, sex, and psychological factors an interaction term was created with GCPS status at baseline and fatigue or fatigue score to determine whether this covariate modify the RR associated with fatigue. In both analyses (Aims 1 and 2), the relative risk (RR) and their 95% confidence intervals (CI) were estimated. In the final multivariable models, we kept in the model fatigue, the covariates associated with the dependent variable, and the effect modifiers (interaction). The likelihood ratio test was used to assess the significance of the RR and the interactions in the model. All analyses were performed using the statistical software package SAS (SAS 9.4; SAS Institute, Cary, NC, US), with the significance level for type I error set at the 0.05 level.
Secondary analysis
Interaction terms were created between fatigue and acute-chronic pain status and dysfunction status both at baseline, to determine whether fatigue’s risk depended on these covariates. The interaction term was retained in the model only if the significance level of the regression coefficient was equal to or lower than 0.10. Further, the analyses were stratified by pain duration (acute [≤ 3 months], chronic [> 3 months]) and dysfunction (no [GCPS I] and yes [II-IV]).
Results
Description of the baseline acute and chronic cohort defined by pain duration
A total of 514 subjects were informed about the study. Of these, 10 refused to participate (lack of time and distress), and 50 were not eligible. Table 1 shows the baseline characteristics of acute and chronic TMD-related pain cohorts defined by duration.
From a total of 454 TMD-related pain subjects recruited, 123 (27.09%) were included in the acute cohort (≤ 3 months) and 331 (72.91%) in the chronic (> 3 months). The chronic cohort included a larger number of subjects with fatigue (48.34%) and females (78.32%) than the acute cohort (35.77%, P = 0.02, 69.40%, P= 0.04).
From 454 subjects enrolled, 376 (82.28%) completed the 3-month follow-up. The chronic cohort included a larger number of subjects with fatigue (50.0%) than the acute cohort (34.31%, P = 0.007) (Table 2). From the acute TMD-related pain cohort including 102 subjects who completed the 3-month follow-up, 50.98% (n = 52) of subjects presented a transition to chronic TMD-related pain, and 49.02% (n = 50) of subjects had no pain. From the 274 chronic TMD-related pain cohort, 75.91% (n = 208) subjects had persistent chronic TMD-related pain whereas 24.09% (n = 66) of subjects had no pain, at 3-month follow-up.
Table 3 shows the baseline profile of subjects with (n = 260) and without chronic TMD- related pain (n = 116) at a 3-month follow-up. Fatigue was present in 49.62% of the chronic TMD- related pain subjects, and in 37.07% of the subjects without pain at 3-month follow-up (P = 0.02).
Table 2:Baseline profile of the acute and chronic TMD-related pain cohorts who completed the 3-month follow-up.

Table 3:Baseline profile of subjects with chronic TMD-related pain and those without pain at 3-month follow-up.

Fatigue contribution to the transition and persistence of chronic TMD-related pain
Table 4 shows the findings of the binary logistic regression analyses. Fatigue at baseline was associated with the transition or persistence risk at 3-month follow-up (RR = 1.17, 95%CI: 1.02- 1.34, P = 0.02). When the model was adjusted by the covariates associated with the study outcome, fatigue RR was weaker and not significant (RR =1.01, P = 0.99).
Table 4:Crude and multivariable logistic regression analyses assessing the contribution of fatigue on transition and persistent TMDrelated pain at 3-month follow-up.

Secondary analyses
No interaction was found between fatigue and acute and chronic TMD-related pain at baseline (P = 0.17). Furthermore, the stratified analyses showed that fatigue was not associated with the transition (RR = 1.22, 95%CI: 0.84-1.75, P = 0.28) and persistent (RR = 0.98, 95% CI: 0.86-1.11, P = 0.78) risks.
Acute and chronic cohort defined by dysfunction
Table 5 shows baseline characteristics of cohorts with and without dysfunction. At baseline, fatigue was present in 50.0% of the dysfunction subjects, and in 33.33% of subjects without dysfunction (P = 0.001). The chronic cohort included a larger number of subjects with psychological symptoms (66.35%) than the acute cohort (47.73%, P = 0.0002).
Table 6 exhibits a baseline profile of the no dysfunction and dysfunction cohorts who completed the 3-month follow-up. Fatigue was more common in the dysfunction cohort (50.38%) when compared to those without dysfunction (33.64%, P = 0.003). Psychological symptoms remained different between these cohorts that completed the follow-up (66.17% vs 51.40%, P = 0.008).
From the no dysfunction baseline cohort including 107 subjects who completed the 3-month follow-up, only eight (7.48%) presented a transition to chronic TMD-related pain defined by dysfunction, and 92.52% (n = 99) of subjects had no transition and remained with no dysfunction. The dysfunction cohort (n = 266) displayed 90 (33.83%) subjects with the persistence of chronic TMD-related pain defined by dysfunction at a 3-month follow-up.
Table 7 shows the baseline profile of cohorts of subjects with or without dysfunction at 3- month follow-up. Fatigue remained to be more frequent among subjects with dysfunction (63.27%) compared to those without dysfunction (39.27%, P <.0001).
Table 6:Baseline profile of the no dysfunction and dysfunction cohorts who completed the 3-month follow-up.

Fatigue contributions to the transition and persistence of chronic TMD-related pain defined by dysfunction
Table 8 displays the findings of the binary logistic regression analysis including 373 subjects. Fatigue at baseline was associated with the transition or persistence risk based on dysfunction (RR crude = 2.05, 95% CI: 1.44-2.94, P < .0001) and remained significant in the multivariable analysis (RR ModelIII = 1.72, 95%CI: 1.21-2.44, P = 0.002) adjusted for dysfunction (RR = 4.07, P < .0001), acute and chronic pain status (RR = 1.27, P = 0.27), and sex (RR = 1.41, P = 0.12). Psychological factors and age were not included in the final multivariable model because they were not associated with chronic TMD-related pain and did not improve the precision of the model.
Table 8:Crude and multivariable logistic regression analyses assessing the contribution of fatigue on the transition to chronic TMDrelated pain based on dysfunction at 3-month follow-up using GCPS.

Secondary analysis
No interaction was found between fatigue and dysfunction at baseline (P = 0.12). The stratified analysis demonstrated that fatigue was associated with increased risk of transition from acute to chronic TMD-related pain (RR = 5.92, 95% CI: 1.25-27.85, P = 0.02), and with the persistence of chronic pain (RR = 1.62, 95% CI: 1.14-2.30, P = 0.007).
Crude (RR crude analysis = 1.03. 95% CI: 1.02-1.05, P < .0001) and the multivariable analysis adjusted for acute and chronic pain status, sex, psychological factors and clinically significant pain and dysfunction at baseline (n = RR multivariable model = 1.01. 95% CI: 1.00-1.03, P < .002) demonstrated that fatigue score was associated with an increased risk of transition and persistent at a 3-month follow-up. An interaction was found between fatigue score and dysfunction at baseline (P = 0.06), suggesting that the RR of fatigue score is modified by dysfunction at baseline. Based on stratification analyses, the fatigue score was also positively association with no dysfunction (RR = 1.02, 95% CI: 1.01-1.04, P < .0001) and with dysfunction (RR = 1.03, 95% CI: 1.01-1.03, P < .0001), regardless of acute and chronic pain status at baseline and sex.
There were no statistically significant differences between subjects who dropout (n = 78) and who did not (n = 376): fatigue (P = 0.45), acute to chronic (P = 0.97), dysfunction (P = 0.45), psychological factors (P = 0.18), age (P = 0.35), sex (P = 0.46), and CPI (P = 0.81).
Discussion
The primary finding of this prospective 3-month cohort study is that fatigue was associated with the risk of transition and persistent TMD-related pain, at a 3-month follow-up when chronic pain is defined by dysfunction (GCPS II-IV). Further, this association was not confounded by subjects’ age, sex, psychological symptoms, acute and chronic pain status and dysfunction at baseline (Table 8). Additionally, fatigue risk was not modified by the acute or chronic pain status risk. Finally, the risk of transition and persistence of chronic TMD-related pain was positively related to fatigue score. Fatigue, however, was not related to the transition from acute to chronic TMD-related pain risk when chronic pain is defined by pain duration (Table 4). A search of the literature revealed nil studies examined the contribution of fatigue on the transition from acute to chronic TMD-related pain as well as its persistence. Chen et al. diagnosed 159 TMD pain patients using a modified version of the RDC/TMD criteria from the orofacial pain clinic and found 10% report having fatigue [3]. Dahan et al. in a cross-sectional study found a fatigue prevalence of 14.4% in their chronic myofascial TMD pain population [4]. In our study, fatigue was reported by 35.77% of the acute TMD-related pain cohort, and 48.34% of the chronic cohort. These percentages of fatigue are close to those observed by Hoffmann et al. who surveyed 1511 TMD-related pain patients and found that 42-43% reported having fatigue after the onset of TMD pain [9].
We investigated if the risk would be confounded by the potential confounders such as age, sex, psychological factors (anxiety and depression), CPI, acute and chronic pain status, and dysfunction at baseline, on the fatigue’s risk. Our baseline findings show dysfunction (GCPS II- IV) present in the chronic (n = 232, 72.96%) compared to acute (n= 86, 27.04%). Similar findings were found of dysfunction distribution among acute (n = 99, 26.54%) and chronic (n = 72, 73.46) at 3-month follow-up. This is consistent with findings from Garofalo et al. [8] showing dysfunction prevalence of more than 70% at baseline at 6-month follow-up. In this study, the mean CPI at baseline was also higher in individuals of chronic TMD-related pain at 6-month follow-up in comparison to those with CPI means of less than 15.
The mean age and sex distribution of our subjects at baseline were similar to Garofalo et al. [8]. Garofalo et al. [8] also found that CPI of chronic TMD-related pain increased the risk of transition from acute to chronic pain at 6-month of follow-up. In addition, a borderline association risk was found with disability (GCPS III-IV) [8].
Regardless, although causality cannot be evaluated due to the design of the present study, these findings raise the hypothesis that fatigue may contribute to a dysregulation of pain modulatory systems involving central and peripheral sensitization that contribute to the transition and persistence of chronic pain defined by dysfunction [22].
The findings of this study are strengthened by the strong methodology utilized and the characteristics of the study population. This is a prospective cohort study that ensures that the risk factors preceded the transition and persistence of chronic TMD-related pain. Prospective cohort studies are considered the gold standard of observational research [17]. Subjects were recruited from four different dental clinics, which decreased the chance of finding a positive association specific to a given hospital because of a referral pattern. Patients were diagnosed by four investigators following the same protocol to decrease misclassification. The questionnaires used are validated and have adequate specificity and sensitivity.
It is important to bear in mind that even though this study has several strengths, it also has a few limitations. First, the classification of acute and chronic TMD-related pain has been used differently among researchers. To avoid misclassification, we followed the IASP to classify chronic pain, which suggested more than 3 months. GCPS is a validated instrument that we used to classify subjects with or without dysfunction [24]. Second, a self-report method was used to collect data. This method might have some disadvantages such as misunderstanding, exaggeration, and/or not remembering some details. Third, the acute cases sample size was not large enough to adequately study the transition from acute to chronic TMD-related pain. This is due to the difficulty of recruiting acute TMD patients due to COVID19 restrictions that were initiated on March 2019 closures. The known number of patients required to recruit enough eligible subjects for the study is not precise. We have conservatively estimated that 80% of patients who meet the criteria will be interested in the study to enable us to meet the sample size. The sample size for acute patients may have been close to achieving our power of analysis but yet was smaller than planned (120 subjects versus 150 subjects). Therefore, type 2 error was introduced, where some results did not reach statistical significance although the hypothesized trend was evident. A larger sample size in the acute cohort may have strengthened the power to demonstrate the associations in this study. This study design also takes time and is conducted at high costs which may be a limitation to some researchers. Finally, a major disadvantage of this type of prospective cohort design is a loss of follow-up. A follow-up rate of 50-80% has been suggested as acceptable by different authors [7]. In our study, our dropout rate is 10.2%. No significant differences were found between subjects who dropped out and did not.
This study has several clinical implications. We found that fatigue contributed to the risk of transition and persistence risk of chronic TMD-related pain when chronic pain is defined by dysfunction. Assessing the patient’s level of fatigue may be introduced into a comprehensive clinical exam protocol to aid in the management of TMD-related pain patients. It is critical to match the level of complexity of the case with the appropriate management program. For example, a patient reporting a single comorbidity will be managed differently from a patient presenting with multiple comorbidities. The latter may require an interdisciplinary pain clinic setting that uses a team of clinicians to address different aspects of the problem in a concerted fashion. Failure to identify and to address the entire scope of the problem may lead to no improvement in pain or function, and further perpetuation of the problem.
In conclusion, this prospective cohort study is the first study to reveal the contribution of fatigue on an increased risk of transition from acute to chronic pain and the persistence of chronic TMD-related pain at a 3-month follow-up when chronic pain is defined by dysfunction. Fatigue did not contribute to an increased risk of transition from acute to chronic, and the persistence of chronic TMD-related pain at a 3-month follow-up, when chronic pain was defined by duration. This result suggests that fatigue assessment should be considered as part of the comprehensive clinical exam for TMD patients and that its management should be tested as potential management to prevent the transition and persistence of TMD-related pain.
Conflict of interest statement
The authors do not have any conflicts of interest associated with this manuscript.
Acknowledgment
This study was funded by Le Réseau en Santé Bucco-dentaire et Osseuse (RSBO) and Quebec Pain Research Network.
References
- (1983) ADA President's Conference sets guidelines for examination, diagnosis and management of TM disorders. Journal (Canadian Dental Association) 49(7): 480-482.
- Truelove EL, Sommers EE, LeResche L, Dworkin SF, Von Korff M (1992) Clinical diagnostic criteria for TMD. New classification permits multiple diagnoses. J Am Dent Assoc 123(4): 47-54.
- Isong U, Gansky SA, Plesh O (2008) Temporomandibular joint and muscle disorder-type pain in U.S. adults: the National Health Interview Survey. J Orofac Pain 22(4): 317-322.
- Von Korff M, Dworkin SF, Le Resche L, Kruger A (1988) An epidemiologic comparison of pain complaints. Pain 32(2): 173-183.
- Slade GD, Bair E, Greenspan JD, Dubner R, Fillingim RB, et al. (2013) Signs and symptoms of first onset TMD and sociodemographic predictors of its development: the OPPERA prospective cohort study. J Pain 14(12 Suppl): T20- 32.e21-23.
- Ohrbach R, Dworkin SF (1998) Five-year outcomes in TMD: relationship of changes in pain to changes in physical and psychological variables. Pain 74(2-3): 315-326.
- Rammelsberg P, LeResche L, Dworkin S, Mancl L (2003) Longitudinal outcome of temporomandibular disorders: a 5-year epidemiologic study of muscle disorders defined by research diagnostic criteria for temporomandibular disorders. J Orofac Pain 17(1): 9-20.
- Aaron LA, Burke MM, Buchwald D (2000) Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med 160(2): 221-227.
- Johnston S, Brenu EW, Staines D, Marshall Gradisnik S (2013) The prevalence of chronic fatigue syndrome/ myalgic encephalomyelitis: a meta-analysis. Clinical epidemiology 5: 105-110.
- Dahan H, Shir Y, Velly A, Allison P (2015) Specific and number of comorbidities are associated with increased levels of temporomandibular pain intensity and duration. Journal of Headache & Pain 16: 528.
- Hoffmann RG, Kotchen JM, Kotchen TA, Cowley T, Dasgupta M, et al. (2011) Temporomandibular disorders and associated clinical comorbidities. Clin J Pain 27(3): 268-274.
- Von Korff M, Ormel J, Keefe FJ, Dworkin SF (1992) Grading the severity of chronic pain. Pain 50(2): 133-149.
- Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, et al. (2015) A classification of chronic pain for ICD-11. Pain 156(6): 1003-1007.
- Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, et al. (2019) Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 160(1): 19-27.
- Dworkin SF, LeResche L (1992) Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord 6(4): 302-355.
- Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, et al. (2014) Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group dagger. J Oral Facial Pain Headache 28(1): 6-27.
- Valko PO, Bassetti CL, Bloch KE, Held U, Baumann CR (2008) Validation of the fatigue severity scale in a Swiss cohort. Sleep 31(11): 1601-1607.
- Löwe B, Wahl I, Rose M, Spitzer C, Glaesmer H, et al. (2010) A 4-item measure of depression and anxiety: validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J Affect Disord 122(1-2): 86-95.
- Chen H, Nackley A, Miller V, Diatchenko L, Maixner W (2013) Multisystem dysregulation in painful temporomandibular disorders. J Pain 14(9): 983-996.
- Dahan H, Shir Y, Nicolau B, Keith D, Allison P (2016) Self-Reported Migraine and Chronic Fatigue Syndrome Are More Prevalent in People with Myofascial vs Non myofascial Temporomandibular Disorders. J Oral Facial Pain Headache 30(1): 7-13.
- Garofalo JP, Gatchel RJ, Wesley AL, Ellis E, 3rd (1998) Predicting chronicity in acute temporomandibular joint disorders using the research diagnostic criteria. J Am Dent Assoc 129(4): 438-447.
- Velly AM, Fricton J (2011) The impact of comorbid conditions on treatment of temporomandibular disorders. J Am Dent Assoc 142(2): 170-172.
- Thiese MS (2014) Observational and interventional study design types; an overview. Biochem Med (Zagreb) 24(2): 199-210.
- Fewtrell MS, Kennedy K, Singhal A, Martin RM, Ness A, et al. (2008) How much loss to follow-up is acceptable in long-term randomized trials and prospective studies? Arch Dis Child 93(6): 458-461.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...