
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4692
Research Article(ISSN: 2637-4692) 
Release of Protein from Teeth During and in Between Treatments with 44% Carbamide Peroxide, 35% Carbamide Peroxide with LED Light, 14% Hydrogen Peroxide and Phthalimidoperoxycaproic Acid Volume 5 - Issue 3
Kelly Keenan*, Luke Ngo, Hari Acharya, Mueed Hossain, Keith Sylvestre and Anand Thakkar
- Chemistry Program, Stockton University, 101 Vera King Farris Drive, USA
Received: March 17, 2022; Published: March 25, 2022
Corresponding author: Kelly Keenan, Chemistry Program, Stockton University, 101 Vera King Farris Drive, Galloway NJ 08205 USA
DOI: 10.32474/MADOHC.2022.05.000211
Abstract
Chemical oxidation of stains using hydrogen peroxide (HP) or carbamide peroxide (CP) is one of several approaches to whiten teeth. There is an increasing number of consumer products with varying concentrations of these active ingredients as well as ways to apply the product. Pthalimidoperoxycaproic acid (PAP) is another oxidizing agent that has recently been used in consumer products. The goal of this project was to measure both the amount of collagen and other proteins released from teeth treated with either 44% CP, 35% CP with LED light, 14% HP or PAP. Products with 35% CP, 44% CP and PAP had short exposure times (30 min) while the 14% HP was left on teeth overnight. Teeth were suspended in artificial saliva (AS) for six days before first round (12 treatments applied as recommended by the manufacturer) followed by another six days with no treatment and a second round. AS samples were collected and a trichloroacetic acid precipitation was done to separate the non-collagen protein from collagen. A modified version of the Lowry assay was done to detect level of collagen. Results indicated that no protein was released from teeth prior to treatment but both collagen and other proteins were released upon treatment with CP or HP but not PAP. The levels of non-collagen and collagen were similar for the 14% HP and 35% CP and both were lower than the 44% CP. For HP and CP, proteins were released between treatments. In addition, the AS samples were concentrated, and proteins were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). There were no proteins visible before treatment and a protein at 72 kDa was released during the first round as well as between rounds. This molecular weight (MW) is consistent with bone acidic glycoprotein. Further treatment resulted in a protein with a MW of 124 kDa which is similar to the subunit for collagen. These results suggest that treatment with CP or HP but not PAP results in loss of both types of protein from teeth and this release can continue even in absence of further treatment.
Keywords: Hydrogen Peroxide; Pthalimdoperoxcaproic Acid; Collagen; Whitening; Carbamide Peroxide; Protein
Introduction
Tooth whitening has become increasingly popular; according to the American Academy of Cosmetic Dentistry, Americans spend 1.4 billion dollars per year on tooth whitening. There are several approaches to whitening including the use of abrasives for mechanical removal of material, colorants, and oxidation of chromophores that make the stains [1,2]. The most commonly used ingredient for the oxidation approach is HP or its precursor—CP. For the consumer, this active ingredient can be administered in a variety of ways including whitening toothpastes, over-the counter strips or in gel form which is placed in a tray. The last method can be used with or without an LED light, which has been shown to increase the efficacy at high concentrations of CP and HP [3].
Generally, these methods are done for short periods of time (30 min or less based on tooth sensitivity). There is also a HP containing pen that contains a solution that is applied to the teeth and left on overnight. In addition to these over-the-counter methods, there are professional treatments done in dentist’s offices where higher concentrations of the active ingredient are generally used. Teeth consist of multiple layers. The enamel is the outermost layer; it mostly non mineral and contains a small number of proteins [4]. Underneath the enamel is the dentin. While less mineralized than enamel, it contains 70% mineral content and 20% organic content or mostly protein. The most common protein is collagen. HP is known to be able to penetrate both the enamel and dentin of tooth [5]. There is greater penetration and higher HP concentrations in bovine teeth that have been demineralized [6].
The efficacy of HP and CP in tooth whitening has been measured in multiple studies [1]. HP whitens teeth by oxidation of chromophores and has also been shown to damage proteins [7]. CP has been shown to deleterious to collagen. There were visible changes in appearance in both collagen scaffolds as well as disks made from teeth treated with CP at 5 or 10% concentration. In addition, there was a loss of Raman spectroscopy signal from amide bonds upon treatment with CP [7]. When protein and collagen levels are directly measured following extraction from 6.5% HP treated teeth, there was a decrease in both when whitening strips. Both protein and collagen were detected in the fluid surrounding treated teeth while none was observed for untreated teeth [8]. The enamel content, measured by thermogravimetric analysis, decreases significantly when treated with 5 or 16% CP [9]. In addition, cell viability for tooth and gum cells decreased when exposed to CP. The effects of HP and CP are not limited to the tooth only and have been shown to damage gums as well. There were increased levels of HP and various proteins involved in the inflammatory response in the gingival crevicular fluid following treatment with 35% CP [10]. PAP is an alternate to CP and HP. When tested on enamel removed from bovine teeth that were stained with red tea, 5% PAP produces whitening equivalent to 3% HP. In addition, while HP produced a visible change in appearance of enamel when viewed with scanning electron microscopy, none was observed for PAP. In addition, there was no change in hardness in PAP-treated for enamel, but it did decrease for HP-treated enamel [11]. PAP is the active ingredient for some commercial products. The amount used is described as proprietary by the manufacturer.
Although proteins in teeth are found in both enamel and dentin, these proteins do not have the same properties. Collagen, the major protein in the dentin, contains a tightly packed structure that is due to covalent cross linking between subunits. This prevents the penetration of the reagents used in the Lowry method—the typical method to measure total proteins. Higher temperatures allow for penetration of the reagents and it is necessary to measure total collagen using a modified version of the Lowry assay [12,13]. Collagen shows another unusual property as well. While most proteins precipitate if a strong acid like trichloroacetic acid (TCA) is added, collagen is an exception. This difference in solubility in TCA is the basis to separate the collagen from the rest of the proteins which are referred to as the non-collagen protein.
The goal of this project was to measure the amount of protein and collagen released from teeth treated with of HP, CP or PAP consumer products and to determine if protein elutes in these teeth even after treatment has concluded. Products contained the following: 44% CP, 35% CP with LED, 14% HP pen and PAP. Teeth were first suspended in a solution of AS for 6 days prior to treatment and then received the first round (12 total treatments). This was followed by an additional six days with no treatment and finally the second round.
Materials and Methods
Treatments of Teeth
In each experiment, four molars extracted by a local dentist from patients that signed an informed consent form were used. All teeth were free of any visible damage. Teeth were washed and placed into pieces of Styrofoam and suspended in 50 ml of artificial saliva (AS); the AS was replaced daily for six days [14]. Manufacturer’s instructions were followed. For the 35% CP sample, a gel was placed in a tray with the attached LED light for 30 minutes. For the 44% CP sample, the molars were placed in a tray with the gel for 20 minutes. For the 14% HP sample, a pen was used to apply the solution to teeth and left on overnight. PAP strips were applied to teeth for 30 min. At the completion of each treatment, the teeth were stored at 37 ºC in AS until the next treatment. The first round consisted of 12 treatments and then teeth were stored in AS for an additional six days at 37 ºC with daily changes of the AS. Afterwards, a second round of 12 treatments was completed. Samples were collected daily and tested for non-collagen protein using trichloroacetic acid (TCA) precipitation, collagen using modified Lowry assay and for size of proteins that eluted using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE).
Measurement of Non-Collagen Proteins
The AS samples were measured for total amount of non-collagen proteins by trichloroacetic acid (TCA) precipitation. Five volumes of AS sample were mixed with one volume of 100% TCA solution and stored at 4 ºC for 10 min and centrifuged in a microfuge at 14,000 x g for 5 min at 4 ºC. The supernatant contained collagen and was collected and tested in the modified Lowry assay. The pellet was washed twice with – 20 ºC acetone and centrifuged at 14,000 x g for 5 min before being dried at 95 ºC for 5 min. The resulting pellet was weighed and used to determine the amount of protein in mg/ml.
Measurement of Collagen Proteins
The supernatant from the TCA precipitation procedure was adjusted to neutral pH and used in the modified Lowry procedure. Gelatin was used as the standard. Briefly, reagent A was added to sample and incubated at 50 °C for 20 min. Reagent B was added and kept at room temperature before adding 1:5 Folin reagent followed by incubation at 50 °C for 10 min. Absorbance was measured on a microplate reader at 600 nm and mg/ml of collagen was calculated based on the standard curve from the gelatin. Values are expressed as the average of six trials.
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis of as Samples
A portion of the AS samples were transferred to Pall nanosep filtration units (MWCO of 3.5 kDa) and centrifuged at 12,000 x g for 2 min at 4 °C. Sample was removed from above the filter, mixed with an equal volume of 2 X sample buffer and heated at 95 °C for 5 min prior to loading on SDS PAGE gels. Bio Rad mini protean gels were prepared according to manufacturer’s instructions: 6% for the resolving gel and 4% for the stacking gel. Sample was loaded as well as Bio-Rad high molecular weight unstained marker. Gels were run at constant 150 V for 45 min prior to staining with Bio Rad Silver Stain Plus according to instructions. Gels were imaged using Kodak imager.
Measurement of Hydrogen Peroxide Concentration
Hydrogen peroxide was measured by titration with potassium permanganate. An appropriate mass of the 30% H2O2 commercial standard, 14% HP and 35% CP were transferred to a volumetric flask and brought up to volume with distilled water. A portion was removed and mixed with water and concentrated H2SO4 using a potassium permanganate solution until the pink color remained. The % H2O2 by weight = (ml KMnO4 * Normality of KMnO4 * 0.010701 * 1000)/g of sample used. Values expressed are average of 8 trials [15].
Results
Hydrogen Peroxide Concentrations
The content of hydrogen peroxide/carbamide peroxide was measured by titration with potassium permanganate. This reaction is based upon peroxide oxidizing the permanganate ion to pink manganese (II) oxide under acidic conditions. In order to test the validity of the method, a commercially purchased 30% hydrogen peroxide solution was used and the results were 29.4% ±0.7 %. These results suggest the method is valid for concentrations used in the treatments in this study. Following the procedure, recommended masses of 35% CP and 14% HP samples were dissolved in water and titrated to measure the peroxide concentrations. The peroxide concentration for the 35% CP sample was determined to be 32.0% ± 0.8%; this value is close to the manufacturer’s claim of 35%. The result also demonstrates that the method works well for CP and is not limited to hydrogen peroxide. The 14% HP same gave a value of 9.2% ± 0.75%. While this value is 66% of the manufacturer’s claim, this sample tended to form a solid when water was added, and it is possible not all HP was extracted into the solution. Overall, the results demonstrate that the levels of CP and HP are consistent with the listed values.
Release of protein
The AS fluid was collected from teeth prior to treatment, during the first round (one round equals twelve treatments), in between the rounds as well as a second round. Both non-collagen and collagen protein levels were measured. The non-collagen proteins were collected by a precipitation with TCA. The highly acidic pH denatures and precipitates most proteins but not collagen which has high solubility at low ph. The collagen amounts were measured in the supernatant using the modified Lowry method. Teeth with no whitening treatment were previously shown to release no collagen or non-collagen protein [6]. These results are consistent with this study where there was no collagen or non-collagen protein released from all samples prior to whitening treatment. The first round of treatment produced a release of non-collagen treatment for the 14% HP that was similar to the 35% CP. Despite the difference in concentration of active ingredient, these treatments are applied for different lengths of time; 14% HP is an overnight treatment while 35% CP is only 30 min. A higher amount of non-collagen protein was released by 44% CP. A previous study using over-the-counter whitening strips which the manufacturer listed as 6.5% HP showed a lower release of non-collagen protein: the value was 0.626 mg/ml [6]. This showed a higher release of protein when higher amounts of CP or HP are used. Unlike CP and HP, there was no release of noncollagen protein from teeth treated with PAP.
Non-collagen protein was released from teeth in the first round and it was also released in between rounds. The amounts released range from 31.9% of the amount released during treatment for the 14% HP to 92.5% of this value for the 44% CP treatment. These results suggest that the treatments alter teeth in such a way that non-collagen protein continues to be released. This release of proteins continues in the second round. For the 14% HP, the amount released in the second round is not significantly different than the first round and it is significantly higher in the second round for the 35% and 44% CP treatments (Table 1). The amount of collagen protein was also measured before, during and in between rounds of treatment of teeth. In order to do so, the supernatant from the TCA precipitation was collected, pH was adjusted to neutral and the modified Lowry assay was done. The results are shown in Table 2. There was no collagen released from teeth prior to treatment but there was a release from teeth treated with CP or HP. In the first round, there was no significant difference in the amount of collagen that left teeth for 14% HP and 35% CP while the amount released from 44% CP was higher. A similar pattern was observed for the non-collagen protein. The values in this study are higher than those observed for treatments with 6.5% HP whitening strips in a previous study: those teeth averaged 0.659 mg/ml collagen. There was no collagen released from teeth treated with PAP. In between rounds of treatment, teeth treated with either HP or CP continued to lose collagen. For 14% HP, the value was 74% of the first round value while 35% CP and 44% CP teeth respectively lost 60% and 54% observed from the first round of treatment. Results from Table 1 demonstrated the loss of non-collagen protein from teeth treated with CP or HP; these results demonstrate the same is true for collagen. Collagen is released during the second round of treatment. The value for the 14% HP and 44% is lower than the first round while it increases for the 35% CP.
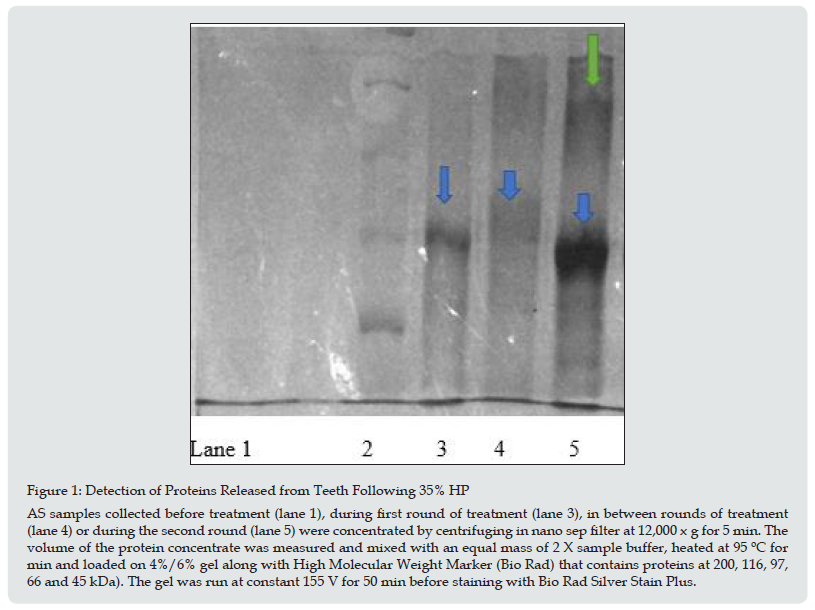
Proteins released from teeth were separated and visualized by SDS PAGE. This method separates proteins based on size of the subunits and the results are shown in Figure 1 for the 35% CP sample. There were no proteins observed in the pre-treatment sample (lane 1) but a 72 kDa eluted from teeth during the first round (lane 3, blue arrow). This protein is similar in size to bone acidic glycoprotein which has a MW of 75 kDa and has been previously observed in teeth [5]. Although not as distinct there does appear to be protein at this MW that eluted from teeth following the first round on treatment (lane 4). This result is consistent with Table 1 which showed non collagen protein eluting from teeth following the first round of treatment even in the absence of additional treatment. There was more protein observed in the second round of treatment (lane 5). The prominent band observed at 72 kDa has a slightly lower MW but is more prominent. It is known that hydrogen peroxide can cause a decrease in the MW of proteins [6]. In addition, there are bands at lower MW which presumably are degradation products. Although not as prominent a band does appear at MW of 124 kDa (green arrow), which is similar to the MW of the collagen subunit observed in type I collagen (129 kDa). This result is consistent with Table 2 which shows the highest amount of collagen released in the second round of treatment with 35% CP. In summary, both collagen and protein are released from teeth treated with HP or CP and amounts increased with the concentration used. There were proteins released whose MW correspond to proteins known to be in both enamel and dentin layer. This release continued even after a round was completed. A newer oxidizing agent, PAP, did not produce a release of collagen or protein. These results are consistent with previous experiments that showed PAP as a safer alternative to CP and HP.
Teeth were suspended in AS which was changed once a day over the course of treatment. There were no treatments for 6 days followed by first round, 6 days with no treatment followed by a second round of treatment. Each round consisted of 12 treatments applied according to the manufacturer’s instructions. The amount of non-collagen protein was measured following a TCA precipitation. Values expressed are the average of 6 trials.
Teeth were suspended in AS which was changed once a day over the course of treatment. There were no treatments for 6 days followed by first round, 6 days with no treatment followed by a second round of treatment. Each round consisted of 12 treatments applied according to the manufacturer’s instructions. The amount of collagen protein was measured on the supernatant, after pH adjustment, following a TCA precipitation. Values expressed are the average of 6 trials.
Figure 1: Detection of Proteins Released from Teeth Following 35% HP AS samples collected before treatment (lane 1), during first round of treatment (lane 3), in between rounds of treatment (lane 4) or during the second round (lane 5) were concentrated by centrifuging in nano sep filter at 12,000 x g for 5 min. The volume of the protein concentrate was measured and mixed with an equal mass of 2 X sample buffer, heated at 95 ºC for min and loaded on 4%/6% gel along with High Molecular Weight Marker (Bio Rad) that contains proteins at 200, 116, 97, 66 and 45 kDa). The gel was run at constant 155 V for 50 min before staining with Bio Rad Silver Stain Plus.
Acknowledgements
The authors would like to thank the patients who agreed to donate teeth as well as the dentists that extracted them. K. K. and L.N would like to thank Stockton University for providing intramural funds to support this research project. The authors would like to thank Matthias Ngo and Winchester Ployratana for providing experimental results.
References
- Eachempati P, Kumbargere NS, Kiran Kumar KS, et al. (2018) Home-Based Chemically-Induced Whitening (Bleaching) of Teeth in Adults. Cochrane Database of Systematic Reviews p. 12.
- Rodrigues de Frietas M, de Carvalho MM, Liporoni PCS, et al. (2021) Effectiveness and Adverse Effects of Over-the-Counter Whitening Products on Dental Tissues. Frontiers in Dental Medicine.
- Kury M, Wada EE, da Silva DP, et al. (2020) Effect of violet LED light on in Office Bleaching Protocols: A Randomized Controlled Clinical Trial. J. of Applied Oral Science 11: 28.
- Jagr M, Eckhardt A, Pataridis S, et al. (2014) Proteomics of Human Teeth and Saliva, Physiol. Res Suppl 1 63: S141-S154.
- Llena C, Oreto Martinez G, Forner L, et al. (2018) Hydrogen Peroxide Diffusion through Enamel and Dentin, Materials 11(9): 1694.
- Briso ALF, Goncalves RS, Da Costa FB, et al. (2015) Demineralization and Hydrogen Peroxide Penetration in Teeth with Incipient Lesions, Brazilian Dental Journal. 26(2): 135-140.
- Redha O, Strange A, Maeva A, et al. (2019) Impact of Carbamide Peroxide Whitening Agent on Dentinal Collagen. J Dental Research 98(4): 443-449.
- Keenan KA, Amoah A, Patel S, Ngo L (2021) Release of Collagen and Protein from Teeth Treated with Over-the-Counter Whitening Strips, Modern Approaches to Dentistry and Oral Health Care 4: 392-400.
- Redha O, Mazinanian M, Nguyen S, et al. (2021) Compromised Dental Cells Viability Following Teeth Whitening Exposure. Scientific Reports 15: 15547.
- Colares VLP, Lima SNL, Sousa NCF, et al. (2019) Hydrogen Peroxide Based Products Alter Inflammatory and Tissue Damage Related Proteins in the Gingival Crevicular Fluid of Healthy Volunteers: A Randomized Trial, Scientific Reports 9(1): 3457.
- Qin J, Zeng L, Min W, et al. (2019) Bio-Safety Tooth-Whitening composite gels with novel pthlamido peroxy caproic acid, Composites Communications 13: 107-111.
- Kiew PL, Don MM Modified Lowry’s method for Acid and Pepsin Soluble Collagen Measurement from Clarias Species Muscles 2: 3.
- Komsa Penkovo Regina, Spirova R, Bechev B (1996) Modification of Lowry’s Method for Collagen Concentration Measurment, J Biochem. Biophys. Methods 32(1): 33-43.
- Leung VWH, Darvell BW (1997) Artificial Salivas for in vivo studies of Dental Materials, J Dentistry 25(6): 475-484.
- com/technical-library/analytical-methods/default.aspx?pid=68&name=Permanganate-Titration.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...






