Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4692
Research Article(ISSN: 2637-4692) 
Comparison of Three Alum Hematoxylin–Harris, Mayer’s, Ehrlich Hematoxylin Using Different Tissues–A Study of 60 Cases Volume 3 - Issue 4
Varun Rastogi1, Nikopol Kashyap1*, Nisha Madhesi2, Nishant3, Ashwini Dayma4 and Jyoti Sharma4
- 1Department of Oral pathology, UCMS college of Dental Surgery, Bhairahawa, Nepal
- 2Lecturer, Department of Oral Medicine, UCMS college of Dental Surgery, Bhairahawa, Nepal
- 3Lecturer, Department of Oral pathology, UCMS college of Dental Surgery, Bhairahawa, Nepal
- 4Lecturer, Department of Community & Preventive Dentistry, UCMS college of Dental Surgery, Bhairahawa, Nepal
Received:June 14, 2019; Published: June 24, 2019
Corresponding author:Dr. Nikopol Kashyap, Department of Pedodontics & Preventive Dentistry, UCMS college of Dental Surgery, Bhairahawa, Nepal
DOI: 10.32474/MADOHC.2019.03.000171
Abstract
Objective: The aim of the study was to visualize and compare different alum hematoxylin and its histo-pathological picture, the clarity of staining and retention of stain inside the various tissues. Materials and Methods: The study included histological sections of four groups-Type I (well differentiated Oral squamous cell carcinoma), II (normal Lymph node), III (normal mucous acini), and IV (Fibroepithelial hyperplasia). The study comprised of 3 solutions-solution 1 (Harri’s hematoxylin), 2 (Mayer’s hematoxylin), 3 (Ehrlich’s hematoxylin). Each group of hematoxylin had sections of four groups. Hence, a total number of 60 sections were made. Results: The analysis of the relationship of the following variables, namely, different alum hematoxylin concluded that Harri’s hematoxylin was superior to Mayer’s and Ehrlich hematoxylin. Conclusion: Our study has shown much promise in exploring Hematoxylin and Eosinstain as a routine staining procedure. Further studies on Hematoxylin and Eosin could open a new horizon in the broad field of laboratory techniques. The persistence and continuing viability and growth of Hematoxylin and Eosin morphology indicates that this simple techniq
Keywords: Incisor relationship anomalies, Three planes of space, Therapeutic choices
Introduction
It is commonplace, virtually platitudinous to say that the practice of histopathology has changed almost beyond recognition during the last three or four decades. It is correct that the scope of pathology has widened and that greater diagnostic accuracy can often be achieved [1]. The contribution of staining techniques to provide contrast to tissues, cells and sub cellular components in bright field microscopy has been remarkable, considering that many of these staining techniques are still widely used for diagnostic purposes more than a century after their introduction. There is probably no other area in cell biology where simple histological techniques have survived, many in their original form, from a period before the current generation of cell biologists were born [2]. It is interesting that in these days of rapidly advancing laboratory technology, the most commonly used stain in biology is based on hematoxylin, a naturally occurring compound derived from the logwood tree hematoxylin campechianum [4].
This dye, arguably first used for histology about 1830, has a long history: it had long been used by the native population in the Caribbean area where the tree grows naturally. Hematoxylin is used most widely in the field of pathology where each working day in laboratories around the world, millions of microscope slides stained with Hematoxylin and Eosin are prepared and viewed by pathologists as part of the diagnostic process. The Hematoxylin and Eosin stain is the stain routinely performed in histology laboratories, but it is just as special as the so called “special stains”. It is a special stain for the nucleus. The stain theory is based on the attraction of appositively charged tissue and dye molecules [4]. The combination of mordant and dye is known as a ‘lake’ and in the case on hematoxylin-mordant such lake are positively charged, behaving as cationic dyes at low pH [5]. Numerous histological and histochemical staining solutions use hematoxylin together with mordanting metals such as aluminum, chromium, iron, tungsten, lead and molybdenum [6]. The staining method involves applications of the basic dye hematoxylin which colors basophilic structures (nucleic acid) with blue-purple hue and alcohol based acidic Eosin Y, which colors eosinophilic structures in varying shades and intensities of pink, orange and red.
Hematoxylin can be used as either a progressive or regressive stain. In progressive staining, a milder form of hematoxylin is used that will only stain the nucleus of the cell and cause the nuclear materials to turn a deeper blue when rinsed in water eg. Gill’s, Mayer’s Hematoxylin. In regressive staining a stronger form of hematoxylin is used that will stain everything on the slide and holds fast to the tissue when rinsed. The tissue is overstrained and then differentiated in a dilute aqueous or alcoholic hydrochloric acid solution to achieve the desired results [4]. The color shifts from blue / purple to salmon pink / red. eg. Harri’s, Ehrlich’s hematoxylin. The strength of the solution is an important property of alum hematoxylin. By changing the strength of the solution to double or triple strength solution will result in excellent and clear nuclear staining but with only short staining time. This short staining time is required when speed and accuracy is essential. Hematoxylin can be ripened either by natural process by exposure to air and light and takes 3-4 months eg. Ehrlich’s and Delafield hematoxylin or by chemical oxidation using sodium iodate eg. Mayer’s hematoxylin or mercuric oxide eg. Harri’s hematoxylin. The use of chemical oxidizing agents converts hematoxylin to hematein almost instantaneously, so these hematoxylin solutions are ready to use immediately after preparation [7].
Harri’s hematoxylin is a modified formula that is non acidified and mercury free. Potassium iodate is used to oxidize the solution. The solutions are used at a low pH to provide very selective nuclear staining. Mayer’s hematoxylin is used because it eliminates the necessity for differentiation and blueing of the section. Sodium iodate is used to oxidize the solution. Ehrlich’s hematoxylin, an alum type of hematoxylin stain, used as a regressive staining method for nuclei, followed by differentiation to required staining intensity: the solution may be allowed to ripen naturally in sunlight or partially oxidized with sodium iodate. Hematoxylin remains the most popular stain in histology. Despite the advent of molecular methods in histology and the replacement of micro anatomical studies with cytological studies and immunohistochemistry, alum hematoxylin are used almost exclusively for nuclear counterstaining in the newer methods. Thus the study was designed to visualize the effects and efficiency of staining by using different types of alum hematoxylin using different tissues.
Material and Method
Materials
The study included histological sections of four groups–Type I (well differentiated Oral squamous cell carcinoma), II (normal Lymph node), III (normal mucous acini), IV (Fibroepithelial hyperplasia). The tissue block was retrieved from the archives of department of Oral and Maxillofacial Pathology, Saveetha Dental College, Chennai India from 2005 onwards. The sections were cut using Leica Semi-automatic microtome (RM2245) and the thickness of sections was 3 microns. The study comprised of 3 solutionssolution 1 (Harri’s hematoxylin), 2 (Mayer’s hematoxylin), 3 (Ehrlich’s hematoxylin). Each group of hematoxylin had sections of oral squamous cell carcinoma (n=5), lymph node (n=5), salivary gland (n=5), fibroepithelial hyperplasia (n=5). Hence, a total number of 60 sections were made.
Methodology
Preparation of staining solution
a) Ehrlich’s hematoxylin [8,9]: Dissolve 2g of hematoxylin in 100ml of absolute alcohol. Then 100ml of distilled water was added, 10ml of glacial acetic acid and 15g of potassium alum with constant stirring. 100ml of glycerin was added to the oxidation process and prolong the hematoxylin shelf life. Natural ripening in sunlight takes about 2 months.
b) Mayer’s Hematoxylin [10,11]: Dissolve 1g of hematoxylin with 50g potassium alum and 0.2g sodium iodate in 1000 ml distilled water by warming and stirring, or by allowing standing at room temperature overnight. 50g chloral hydrate and 1g citric acid were then added, and the mixture was boiled for 5min, then cooled and filtered.
c) Harri’s Hematoxylin [12]: 2.5g hematoxylin was dissolved in 25ml absolute alcohol, and was then added to 50g potassium alum, which has been dissolved in the warm 500ml distilled water in a 2 litre flask. The mixture is rapidly brought to the boil and 125g mercuric oxide or 0.5g sodium iodate is then slowly and carefully added. Plunging the flask into cold water or into a sink containing chipped ice rapidly cools the stain. When the solution was cold, 20ml glacial acetic acid was added, and the stain was ready for immediate use.
Procedure
a. Hematoxylin staining
b. Dewax the section, hydrated through graded alcohol to water
c. Remove fixation pigments
d. Harri’s hematoxylin-5-15 min
e. Mayer’s hematoxylin-5 -10 min
f. Ehrlich’s hematoxylin-30 min-1hr
g. Wash well in running tap water until section blue for 5min
h. Differentiate in 1 acid alcohol for 5-10sec
i. Wash well in tap water
j. Blue by dipping in an alkali solution (ammonia water) followed by 5 min tap water wash
k. Stain in 1 Eosin Y for 1 min
l. Wash in running tap water for 1-5min
m. Dehydrate through alcohols, clear and mount All the stained sections were assessed individually by 3 observers and tabulated. The assessment parameters included are as follows: Epithelium cell membrane, nucleus and cytoplasm staining of stratum basale, spinosum, granulosum and corneum layer were assessed. Connective tissue-collagen fibers, fibroblasts, inflammatory cells, adipocytes, blood vessels, muscle and gland were assessed. The scoring criteria used to grade the intensity of tissue staining as follows:
+++ --> 3, ++ --> 2, + --> 1, - -->-0
Mean scores were estimated from the sample for each study groups. Mean scores were compared between different groups by using either by Mann-Whitney U test α Kruskal Wallis One Way ANOVA followed by Mann-Whitney U test.In the present study, P<0.05 was considered as the level of significance. The statistical analysis software used in the study is SPSS version 13.
Results
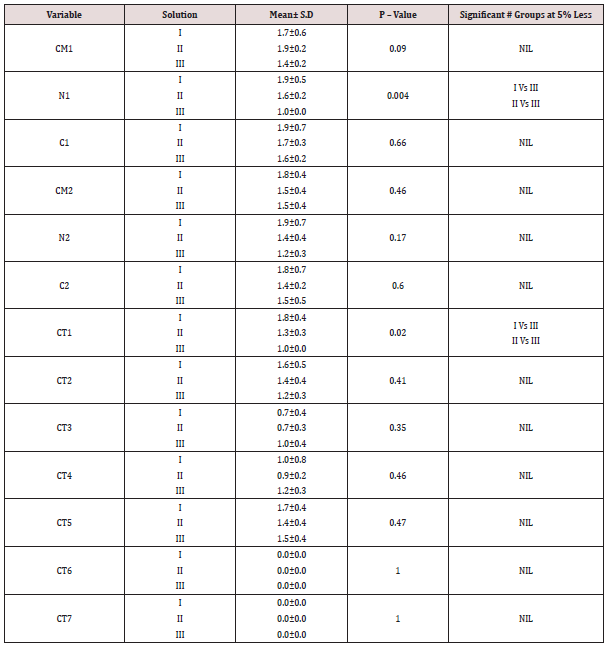
The study included histological sections of four groups-Type I (well differentiated Oral squamous cell carcinoma), II (normal Lymph node), III (normal mucous acini), IV (Fibroepithelial hyperplasia). The tissue block was retrieved from the archives of department of Oral and Maxillofacial Pathology, Saveetha Dental College, Chennai India from 2005 onwards. The sections were cut using Leica Semi-automatic microtome (RM2245) and the thickness of sections was 3 microns. The study comprised of 3 solutionssolution 1 (Harri’s hematoxylin), 2 (Mayer’s hematoxylin), 3 (Ehrlich’s hematoxylin). Each group of hematoxylin had sections of oral squamous cell carcinoma (n=5), lymph node (n=5), salivary gland (n=5), fibroepithelial hyperplasia (n=5). Hence, a total number of 60 sections were made. As per Table 1-4 Comparison of mean scores between different solutions in type I - IV group indicates that Harri’s hematoxylin is superior to Mayer’s and Ehrlich hematoxylin. This is proved further by statistical test (Kruskal-Wallis one way ANOVA and Mann-Whitney U test) which showed that there is a significant difference in mean among the three solution (P<0.05).
Table 1: Comparison of mean scores between different solution in Type I group (oral squamous cell carcinoma).
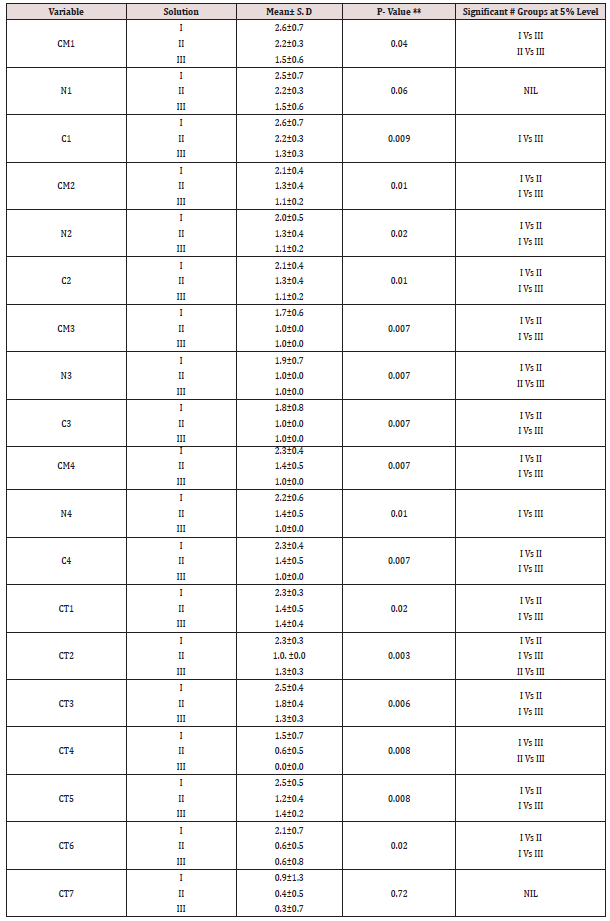
Table 4: Comparison of mean scores among different solutions in Type 4 group (fibroepithelial hyperplasia).

CM1 - Cell Membrane in Basal layer; CM2 - Cell Membrane in Spinosum layer; CM3 - Cell Membrane in Granular layer; CM4 - Cell Membrane in Corneum layer; N1 - Nucleus in Basal layer; N2 - Nucleus in Spinosum layer; N3 - Nucleus in Granular layer; N4 - (Cell Membrane in Corneum layer; C1 - Cytoplasm in Basal layer; C2 - Cytoplasm in Spinosum layer; C3 - Cytoplasm in Granular layer; C4 - Cell Membrane in Corneum layer; CT1 - Collagen fibers; CT2 – Fibroblast; CT3 - Inflammatory Cells; CT4 – Adipocytes; CT5 - Blood vessels; CT6 - Muscle
CT7 - salivary gland.
Discussion
Staining is defined as the visual labeling of some entity by attaching or depositing in its vicinity, a marker of contrast color and shape. Successful histological techniques used for the distinction of tissue components commonly cause two changes in the tissue, either an alteration of contrast or an alteration in colour. Several different types of ‘staining’ process are used to give tissue contrast or colour, usually both, before they are examined with the microscope. Hematoxylin is a naturally occurring chemical used as the basis of dye in laboratories throughout the world to stain nuclei in microscope slide preparation. This chemical is extracted from the logwood tree hematoxylin campechianum a tree of the order leguminosae (genus eucaesalpineae) and so named because of the reddish color of its heartwood (from the Greek Heamoto-blood xylon-wood) and young leaves. [13] The heartwood is very hard and heavy and may range from dark orange to puplish red [14]. Hematoxylin has had several synonyms through history including campeachy wood, block wood and log wood. The crude logwood product also contains tannins, resins, quercitin and a small amount of volatile oil [15].
Hematoxylin can be ripened either by natural process by exposure to air and light and takes 3-4 months to ripen or by chemical oxidation using chemicals such as sodium iodate or mercuric oxide(16).By itself, hematoxylin is amphoteric in its hematein form; it is red at acid pH and blue at alkaline pH. Counterstaining the cytoplasm, if required, adds more tinctorial qualities. The result when viewed microscopically is of a monolayer of cells apparently embedded in a glass. Hematoxylin also is used almost exclusively in cytology laboratories. Although hematoxylin is used widely for routine H&E staining, it is with other staining methods that its versatility is best demonstrated. It can be used for staining a wide variety of tissue structure using a variety of oxidants, mordants and differentiating agents, sometimes followed by counterstains Alum hematoxylins have become the standard, universal means of staining cell nuclei of microscopic examination. Practically every section of normal and diseased tissue will be examined and presumptively identified using an alum hematoxylin to colour nuclei. The major disadvantage of alum hematoxylin as a stain is its susceptibility to acids, which limits the range of counterstains that can be used. Alum hematoxylin staining is also influenced by other factors, including the concentration and age of the staining solution as well as the fixation and processing to the tissue was subjected. Nevertheless, staining with alum hematoxylin is an immensely versatile procedure and with experience, results that are consistent in intensity and effect can be achieved. The alum hematoxylin is routinely used in the hematoxylin and Eosin stain, and produce good nuclear staining. The usual mordant for nuclear staining with hemalum is an alum, or aluminium double sulphate. Ammonium or potassium aluminiumsulphates are the most commonly used alum hematoxylin. The explanation for the use of alums is that they were available in good purity in the late 1800’s when these solutions were being introduced.
The tissues were selected as those that are routinely received to the department of Oral and Maxillofacial Pathology. Normal salivary gland, lymph node, fibroepithelial hyperplasia and oral squamous cell carcinoma were included in the study. The salivary gland mainly the mucous acini being basophilic, can be used to assess the quality of hematoxylin staining. Lymph nodes are mainly made up of lymphocytes and can be used to assess the intensity of hematoxylin staining. In fibroepithelial hyperplasia, the epithelium being hyperplastic can be used to judge or compare the background staining with the hematoxylin staining. In oral squamous cell carcinoma the epithelium being dysplastic, can be used to assess the degree of differentiation and intensity of hematoxylin staining by visualizing the staining of hyperchromatic nuclei, pleomorphism and nucleus/ cytoplasmic ratio.
The hematoxylin and hematein differ by only one hydrogen atom. Removal of this hydrogen from hematoxylin is accomplished either naturally by atmospheric oxygen or by using mild oxidizing agents and results in a compound with a hydroxyl group adjacent to a carbonyl group. Thus, the aluminum in the hematoxylin solution may be perceived as a link, or bridge, between the anionic dye hematein and a negatively charged nuclear phosphate group. Coordination complexes are formed with a covalent bond between the carbonyl oxygen and the same aluminum atom. In this way, the aluminum is firmly attached to the molecule by a process called chelation; therefore, the compound sometimes is called a chelate. However, since a dye is involved, it is more commonly called a lake. It is the lake, which is the staining component of hemalum solutions. The simplest view is that it reacts as cation and attaches to tissue anions, such as phosphate groups of DNA and carboxyl group of proteins. The basic principle involves oxidation of hematoxylin to hematein, which is anionic form, hence cannot stain the tissue. Hematein is then combined with a mordant to convert anionic form to cationic form which ultimately results in staining of tissues. This oxidation of hematoxylin to hematein can occur naturally by exposure to air and light or can be done using chemical agents such as sodium iodate, mercuric oxide etc. Prolonged oxidation of hematoxylin forms intermediate compounds di and tri oxy hematein that may interfere with staining.
Marshall and Horobin 1972 found that oxy hematein is a carboxylic acid and at least one break in the linkage region between the aromatic and quinonoid ring must occur during the oxidation of hematein. This oxy hematein gives a orange yellow color to the tissues [17,18]. The affinity of the stain to the tissue depends upon the concentration of the dye and the amount of mordant in the staining solution. If the mordant is more than the dye in the staining solution, the stain will bind firmly to the tissues and the bond between the stain and tissues cannot be broken easily. If the amount of dye is more than the mordant, the stain binds loosely to the tissues and the bond between the stain and tissue is easily broken. Harri’s hematoxylin has a high mordant content whereas Mayer’s and Ehrlich hematoxylin has a high dye content, hence Harri’s hematoxylin binds firmly to the tissues and results in enhanced nuclear staining. Two types of bonds are involved in the fundamental reaction between a mordant dye and a mordant. One is a covalent bond with hydroxyl oxygen and the other is a coordinate bond with oxygen (the electron donor). The covalent bond forms between the hydroxyl oxygen and the metal and coordinate bond forms between the double bonded oxygen and the metal. Since aluminum have valences of three, it is possible that three molecules of dye could attach to each atom of the mordant metal and attachment to the tissue is also by means of the mordant metal. It is often remarked that the addition of a mordant to an appropriately dye solution result in a very sudden, dramatic change in color. This is due to the incorporation of metal atom into the delocalized electron system of the dye. Metals have relatively low energy levels, so their incorporation into a delocalized system results in a lowering of the overall energy.
When dyes using aqueous solution stains tissue, affinity is facilitated by hydrophobic bonding. When water content is less, second law of thermodynamics comes into play which states that system change spontaneously to maximize its disorder, resulting in dispersion of dye and hence staining occurs [19]. The surface properties of lipids will determine their permeability either to aqueous reagents or to organic solvents. Lipid droplets such as those in storage cells exists as entirely separate entities in a continuous phase, the droplets consist solely of lipid, the surface of which is hydrophobic. Where the lipid is combined with non-lipid elements such as water or protein, the phase is not continuous. In these cases, the surface may be hydrophilic. That means that their surface tension at a lipid- water interface makes them assume a globular shape in aqueous solution. DNA, RNA, and phospholipids are acidic due to their phosporyl groups, and mast cells, cartilage, and some mucous secretions of glands contain acidic sulphuryl and carboxyl groups. Collagen, Red blood corpuscles, and the granules of eosinophil, leucocytes are basic due to the predominance of basic amino groups [20]. The attachment of the mordant metal to tissue is by chelation-covalent and coordinate bond formation. Phosphate hydroxyl groups of the nucleic acids provide means for covalent bonding, and other atoms in the vicinity can donate electrons for the coordinate bond. The DNA strand has a repeating sequence of phosphate and deoxyribose with a base attached, the bases pairing up in the complimentary manner of G≡C and A=T. Attachment of the mordant dye is due to the mordant forming a chelate with the phosphate hydroxyl and another atom in a manner very similar to that between the mordant and the dye.
The intensity and ‘fastness’ or grip of a stain depends upon the avidity of its ionized radicals for tissue components and from the number and strength of these chemical bonds. Carboxyl groups are only weakly acidic whilst phosphoryl groups are stronger and the sulphuryl groups are stronger still; thus sulphonated dyes have strong affinity for the basic groups of proteins, which are in plentiful supply, and will even displace less avid dyes that have already combined with the tissues, thereby staining or differentiating the first dye. The amount of dye ion binding to a tissue substrate depends not only on the charged signs of dye and tissue but also on their magnitude, on the amount of non-dye electrolyte present in the dye bath and the ability of the tissue substrate to swell or shrink. Electrostatic attractions are probably important in pulling dye molecules towards oppositively charged parts of tissue. Ionic bonds may be the only forces holding dye to substrate when staining is by a dilute solution of cationic or anionic dyes with small molecules [19]. The Van der Walls forces are the electrostatic attractions that always exist between the electrons of one atom and the nucleus of another. These occur between all reagents and tissue substrates, but since molecules with extensively delocalized electronic systems tend to have larger dipoles and be more polarizable, Van der Wall’s forces are more important when tissues or stains contain such moieties [19].
The occurrence or non-occurrence of staining by a dye is determined by the thermodynamic principles and by formation of chemical bonds that vary with the different types of dye and substrate. An approximate indication of particle size is given by molecular weight of the dye. However, most dye molecules have a strong tendency to stick together and form aggregates. Large dye particles diffuse in and out of a fibre or a component of a tissue more slowly than small ones. Alum solutions are acidic, a 0.2 molar solution of potassium alum has a pH of 3.3 and at this pH the lake is soluble. As the solution is used and alkaline tap water is introduced into the solution, pH rises until the lakes begin to precipitate. Staining depends largely on the attachment of dye to proteins. These have both positively and negatively charged groups (carboxyl and hydroxyl groups). An observed effect from the addition of acid is that nuclear staining is sometimes more selective. This shows up more obviously with those hemalums having lower dye content. If the pH is raised above the isoelectric point, then the number of charged amino group is reduced and the number of charged carboxyl and hydroxyl group increase causing it to behave as an acid protein. As the pH is lowered from the isoelectric point the number of charged amino groups will increase and the number of charged carboxyl and hydroxyl group will decrease causing the protein to behave as basic protein. The improved nuclear selectivity is probably due to a slight lowering of the pH when extra acid is added. This may be sufficient to eliminate some of the reactions with acidic groups of cytoplasmic proteins. Hematein can participate in non-ionic attachment of dyes to the tissues, and the presence of salts can promote these dipoledispole interactions.
Unfortunately, affinity for Al3+ or the alum hematein complex does not account for the selectively of the nuclear staining by the commonly used hemalum solutions. This is inhibited only slightly by prior extraction of DNA from the tissue, but it is considerably reduced after chemical blocking of lysine and arginine residues. These observations suggest that the dye-mordant complex attaches mainly to a component of the nucleus other than DNA. The nonDNA component of chromatin is the basic nucleoprotein, which would be expected to bind an anion rather than a cationic dye-metal complex. Horobin (1998) suggested that dye-metal complexes are bound to chromatin by both ionic and nonionic forces. The latter are likely to be enhanced by the other substance present in alumhematein staining solutions. Most formulations contain a highly polar substance such as glycerol, ethylene glycol or chloral hydrate, which would be expected to associate by hydrogen bonding with hydrophilic components of the tissue and to interfere with short range forces (Vander walls, hydrophobic etc) that would hold the dye-metal complex to some potential substrates. A shape of the dyemetal complex ion that favorably conforms to the nucleoprotein and nucleic acid molecules would be bound closely enough to resist disruption by hydrogen bond substance.
Solutions containing oxidized hematoxylin (hematein) and Al3+ are the ‘H’ of H & E and are used everywhere to stain the nucleus of cells. The reactions of aluminum ions with hematein have been studied by Bettinger and Zimmermann (1991) who found that a cationic dye-metal complex was present in acid solution [21,22]. The complex was bound by DNA in section of tissue, even though the pH was lower than that at which nucleic acids can be stained by ordinary catonic dyes. The hemalum mixtures in common use contain a large excess of Al3+ ions over hematein molecules. Aluminium ions have considerable affinity for DNA and can prevent its subsequent staining by basic dyes. Acids used to increase the selectively of nuclear staining probably disrupt the bonding between Al3+and parts of tissues other than chromatin, rather than between Al3+ and the dye. Stain - tissue affinities and numbers of binding sites present in tissues can both vary in staining system forming covalent bonds; reagents give colored products only with a limited range of tissue chemical groupings. An understanding of staining system often requires consideration of patterns of affinities. The negatively charged acid dyes have high affinities for tissue structures carrying cationic charges, but low affinities for structures carrying negative charges such as those rich in sulfated glycosaminoglycans or phosphated nucleic acids. Affinities are also influenced by varying the concentration of inorganic salt present. The alum hematoxylins differ primarily in their inorganic salt present.
Conclusion
onclusion Our study has shown much promise in exploring Hematoxylin and Eosinstain as a routine staining procedure. Further studies on Hematoxylin and Eosin could open a new horizon in the broad field of laboratory techniques. There is, therefore, a pressing need for additional research and a large sample size in this area before the true clinical value of Hematoxylin and Eosin as a stain can be determined. The persistence and continuing viability and growth of Hematoxylin and Eosin morphology indicates that this simple technique continues to meet most of the requirements of not only the pathologists but also clinicians, and, let us not forget, patients.
The study concluded that Harri’s hematoxylin was superior to Mayer’s and Ehrlich hematoxylin (p<0.05). The possible reason for this significance was that Harri’s hematoxylin has a more mordant content and less dye, hence the hematoxylin binds firmly to the tissues and results in enhanced nuclear staining in comparison to Mayer’s and Ehrlich hematoxylin, which has a more dye and less mordant content and binds less firmly to the tissue.
References

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...