
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4749
Research Article(ISSN: 2637-4749) 
Occurrence of antibiotic resistance Zoonotic Salmonella isolated from Table Egg in North West Ethiopia Volume 4 - Issue 1
Birhan Agmas1*, Semahegn Yilkal2 and Habtamu Tassew1
- 1Department of Veterinary Science, College of Agriculture and Environmental Science, Bahir Dar University, Bahir Dar, Ethiopia
- 2Agricultural Development Office, College of Agriculture and Environmental Science, Bahir Dar University, Bahir Dar, Ethiopia
Received: August 09, 2020; Published: August 26, 2020
Corresponding author: Birhan Agmas, Department of Veterinary Science, College of Agriculture and Environmental Science, Bahir Dar University, Bahir Dar, Ethiopia
DOI: 10.32474/CDVS.2020.04.000178
Abstract
Salmonella is one of a major foodborne pathogen worldwide that table egg as one of the vehicles for its transmission. Across sectional study was conducted from November 2018 to April 2019 in Bahir Dar Town to identify Salmonella isolates from table egg and to determine their antimicrobial resistance profile. A total of 157 egg samples were collected by systematic random sampling technique and processed for isolation of Salmonella by conventional bacteriological methods. Antimicrobial sensitivity test was performed for 10 selected antibiotic discs by disc diffusion. The overall level of Salmonella prevalence in current study was 2.23 %. Prevalence of salmonella based on site and part of eggs were 3.85% from the open market and 1.28% from the poultry farm. About 3.80% of Salmonella was observed from internal contents of farm eggs. Isolation rate of Salmonella between sample sites and egg parts were not statistically significant (p = 0.69) and (p=0.702) respectively. All Salmonella isolates were susceptible to gentamycin; however, all of them were resistant to ampicillin, tetracycline and cephalothin. All isolates were developing multiple drug resistance. Confirmation of Salmonella from egg indicates hygiene protocols should be applied from farm to mouth to prevent the infection of human beings by Salmonella. Even though the isolation rate was low in current finding, isolates were developing multidrug resistance. Drug resistance is mobile; the genes for resistance traits can be transferred among bacteria of different taxonomic and ecological groups by means of transduction, conjugation, and transformation. Therefore, proper egg handling, strict biosecurity measures and rational antimicrobial usage should be practiced.
Keywords: Antimicrobial Sensitivity; Bahir Dar; Chicken Egg
Introduction
Eggs are nutritious foods that are used throughout the world FAO [1]. The whole egg contains water (75%), proteins (12%), lipids (12%) and carbohydrates and minerals (1%). It is considered a good source of nutrients such as protein, lipids, vitamins and minerals Réhault-Godbert et al. [2]. They have pharmaceutical uses by acting as an anticancer agent, as an immune-modulator and the ability to suppress tumor cells, and have also antiviral agent Abeyrathne et al. [3]. However, eggs are nutritious and have a risk of food-borne illness if we are not maintaining food safety from farm to consumption. Salmonella is one of the causes of food-borne infection associated with egg origin foods worldwide Galiş et al. [4], U.S. Food and Drug Administration, [5]. Salmonella has been isolated from table eggs and egg foods. Nutritional constituents and water content available in table egg and egg foods have become useful growth ingredients for the proliferation of Salmonella. Salmonella, at room temperature highly proliferate and pathogenicity of the organism remained high and active in table egg Whiley and Ross & Long et al. [6,7]. The two most commonly identified serotypes that cause food-borne salmonellosis are: S. enterica serotype Typhimurium and S. enterica serotype Enteritidis. Both serotypes have the ability to colonize the reproductive organs of hens and transmit to humans through raw and under-cooked egg consumption Alghoribi et al. [8]. Salmonella Enteritidis is more commonly associated with contaminated eggs Jajere [9]. It may be transferred to the human due to the poor hygienic conditions and raw eggs/under cooked eggs consumption Whiley and Ross [6].
The prevalence of Salmonella in table egg has been studied in
different regions of the world and health risks were evaluated. In
spite of steady observation and in depth efforts, food-poisoning
flare-ups due to salmonellosis are expanding in western nations
and egg items account for a critical parcel of reported flare-ups. In
developing countries such as Ethiopia, there’s no such ceaseless
observing framework and the precise number of cases isn’t known.
Consequently there is need to study Salmonella occurrence and
antimicrobial susceptibility profile in poultry table eggs in Ethiopia.
Antimicrobial resistance is a global problem due to the
widespread and inappropriate use of antibiotic drugs in food
animals Ayukekbong et al. [10]. Egg may contain drug residues by
laying hens treated with pharmaceutical products Mund et al. [11].
Many drugs used in veterinary medicine have identical analogs
that are used in human medicine Smith et al. [12]. Thus table
egg can establish reservoirs of antimicrobial resistant bacteria
those has veterinary and public health threat Afema et al. [13].
Studies conducted in Ethiopia suggest an increase in antimicrobial
resistance of Salmonella to commonly used antimicrobials in both
public health and veterinary sectors Beyene, Sibhat, Tesfaw &
Dagnew [4,14-16].
Despite the high risk of egg-related salmonellosis, studies
related to its epidemiology in Ethiopia are not adequately addressed
and are not available in the study area. Thus, the aim of this study
was to evaluate the presence of Salmonella in table eggs in North
West Ethiopia. In addition, the antimicrobial susceptibility patterns
of these isolates were evaluated to provide information for future
intervention measures.
Materials and Methods
Description of Study Area
The study was carried out in the town of Bahir Dar, approximately 580 km north-west of Addis Ababa; Ethiopia. Geographically, Bahir Dar is located at latitude 11.59° North and 37.39° East. Its average elevation is estimated to be 1810 m above sea level. As the National Meteorological Agency reported in 2016, the town’s mean annual temperature was 20.85 °C Abebe [17]. According to the Central Statistical Agency 2013, the total population of Bahir Dar Town is 202,157 [18]. According to Bahir Dar Town Agricultural Office’s annual report, there were 13 intensive poultry farms and 36,870 layers in the town [19].
Sample Size Determination
The sample size required for this study was determined based on the single population proportion formula Thrusfield, [20]. There was a previous study on Salmonella in chicken eggs of 11.5 % expected prevalence with 95 % desired confidence interval and 5 % absolute precisions were used. Based on the above formula, the total sample size was 157 eggs. Since the egg sampled internal content and surface, over all 314 samples were processed for microbiological isolation.
Sampling Method
A systematic random sampling technique was used to sample.
The 157 chicken table eggs collected from Bahir Dar Town poultry
farms and markets were taken from November 2018 to April 2019.
Eggs were collected aseptically, individually packed with a sterile
plastic bag. The eggs were transported to Amhara Public Health
Institute Microbiology Laboratory immediately after collection.
Sterile plastic bags containing selected eggs were opened in the lab
and samples were processed immediately with in the safety hood
(Biobase II). The shell surface of intact eggs was sampled by swab
technique. After sterile cotton swabs dipped in sterile buffered
peptone water (BPW), the entire surface area of the egg shell was
swabbed gently. The swab was inoculated directly into screwcapped
bottles containing 10ml BPW [21].
The same eggs from which the shell sample was collected were
also used for egg content sampling. To sample the internal contents,
the surface of the eggs were sterilized by immersing in a solution
prepared from 70 % alcohol and iodine in 3:1 ratio for 10 seconds,
air dried in the safety cabinet for 8-10 min and then cracked with
a sterile knife. An automatic vortex mixer was used to mix well
with egg contents. After each egg content was homogenized, 25ml
of mixed egg content was inoculated into 225 ml of BPW and
homogenized for a two-minute period.
Isolation of Salmonella
Isolation was conducted based on conventional methods for
Salmonella detection, following standard ISO 6579 guidelines
Mooijman et al. [22]. Egg surface swabs were inoculated directly
into screw-capped bottles containing 10ml Buffered Peptone
Water (BPW) and incubated at 37°C for 16-18 hours. Each egg’s
content was thoroughly mixed by using an auto vortex mixer and
25 ml of mixed egg content was inoculated into 225 ml of BPW
and incubated at 37°C for 16-18 hours Suresh et al. [21]. The preenrichment
broth after incubation for 16-18 hours was mixed and
0.1 ml of the broth was transferred into a tube containing 10 ml
of Rappaport-Vassiliadis medium (RV broth). Another 1ml of preenrichment
broth was transferred into a tube containing 10ml
of Muller-Kauffmann tetrathionate broth (MKTT broth). The
inoculated RV broth was incubated at 41.5°C ±1°C for 24 ± 3 hours.
The inoculated MKTT broth at 37 ° C ± 1 °C for 24 ± 3 hours Suresh
et al., [21].
A loop full of RV and MKTT broth inoculums was transferred
and streaked separately onto the surface of Xylose Lysine
Deoxycholate agar (XLD agar); Titan Biotech Ltd., Bhiwadi, India)
and Salmonella-shigella agar (SSA agar; Titan Biotech Ltd., Bhiwadi, India) separately. The plates were incubated at 37°C ± 1°C for 24±3
hours. After proper incubation, the plates were examined for the
presence of suspected Salmonella colonies. XLD agar is pink with a
darker pink center whereas lactose-positive Salmonellae are yellow
with or without blackening. Confirmation was done by using a
biochemical test according to ISO 6579 Mooijman et al. [22].
Biochemical Confirmation of Salmonella Isolates
Each identified colony with typical Salmonella morphology was confirmed biochemically by inoculation into triple sugar-iron agar, methyl-red-Voges-Proskauer broth, indole test, Simmons’ citrate agar, urea agar, lysine iron agar, kligler’s iron agar and SIM medium and incubated at 370C for 18 to 48 hours. The result was interpreted according to International Organization for Standardization guidelines (ISO-6579-1, 2017).
Anti-microbial Susceptibility Testing
Antimicrobial susceptibility test was performed for all Salmonella isolates based on the criteria of the Clinical and Laboratory Standards Institute [23] for Enterobacteriacea. For the susceptibility test, the anti-microbial was selected based on groupings of antimicrobial agents with United States food and drug administration which are commonly used for the treatment of Salmonella in animal and human in Ethiopia. The 10 antimicrobial anti-microbial susceptibility tests were carried out by Kirby- Bauer disk diffusion method on Mueller-Hinton agar (MHA). The inhibition zone was measured with millimeter caliper meter (mm) and interpreted according to the CLSI guideline standard and manufacturer’s recommendation as susceptible, intermediate or resistant Table 1 CLSI [23].
Data Analysis
All data were entered into a Microsoft Excel spreadsheet and transferred to SPSS version 20 for analysis. Salmonella prevalence by source of samples and sample types was expressed as percentages. Descriptive analysis of the collected data was done for most variables in the study using statistical parameters such as percentage and mean. The association was identified by the chisquire test and P-value less than 0.05 were taken as the cut point of significant.
Results and Discussions
Prevalence of Salmonella
Out of 314 samples processed (157 egg surfaces and internal contents each); Only 7 samples, 2.23 %, were Salmonella positive. Of the seven Salmonella positive samples, four isolates were from egg shall surface. The remaining three isolates were from egg internal content samples collected from small scale poultry farm (Figure 1). However, all samples collected from the egg contents of market eggs were negative for salmonella. The overall Salmonella prevalence levels of 2.23 % on eggs in the current study were in agreement with the finding of salmonella species recovered from table eggs in Denmark, with a prevalence of 2.6 %. Another study agreed with our finding was 2.7 % overall prevalence of Salmonella from chicken table egg in Harmaya district, Ethiopia Kemal et al. [24].
Figure 1: prevalence of Salmonella from farm, market and
part of egg types in 2018/19.
ICF= Table egg internal contents from farm, SSF= Table egg
surface swab from farm, ICM= Table egg internal contents
from market, SSM= Table egg surface swab from market
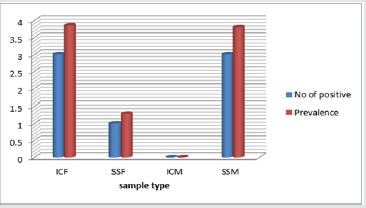
The total isolation rate in the current study was 2.23 % higher
than the study done in retail eggs samples in New Zealand 1.8 %
and from retail egg samples reported in the United Kingdom 0.34
% (Authority, [25]), isolated Salmonella species. A recent survey
of retail eggs from 5,000 egg samples in Northern Ireland and the
Republic of Ireland found only 0.04 %, all of which were lower
than the current study (Murchie et al., [26]). On the other hand, the
current finding was lower than previous studies by Suresh et al. [21]
Reported in South India: 7.7 % Salmonella prevalence in retail eggs
and Tsegaye et al. [27] 13.3 % prevalence reported of exotic chicken
eggs in Alage, Ziway and Shashemene, Ethiopia. Ejo et al. [28] also
reported an 18 % prevalence of raw egg collected from restaurants,
hotels, cafeterias pastry and retail shops in Gondar, Ethiopia. The
probable reason for this variation in Salmonella prevalence could
be due to difference in Salmonella disease epidemiology, difference
in application of good egg handling practices, variation in hygienic
farm situation, store retailers and lack of awareness of Salmonella
status in chicken eggs. Poultry feeds and hatcheries could also be
the reason for the variation in overall prevalence. Kassahun et al.
[29] stated that the presence of chickens of different ages on the
farm, the presence of arthropod pests, wet and soiled litter on the
farm, the housing system and flock size could be important reasons
for egg contamination with various micro-organisms.
Salmonella isolation rate on surface swabs of farm table eggs
in this study was 1.28 % lower than reported by Nguse et al. [30]
in Mekele, Ethiopia, which were 4 %. Kemal et al. [24] on the
other hand did not isolate any Salmonella on the surface of the
Harmaya District poultry farm egg. The prevalence difference may
be due to Salmonella. The prevalence of eggs in this study may be
explained by environmental contamination during storage and
transportation and ineffective cleaning of eggs. Furthermore, the
management system in practice could also be the probable reason
for the variation in prevalence. Salmonella isolate levels were not
varied among sampling sites and types. There was no significant
difference in the level of Salmonella positivity in local markets and
poultry farms (p=0.69) (Table 2).
Table 2: Distribution of Salmonella prevalence based on sampling sites and part of egg in Bahir Dar Town, 2018/19.

The level of Salmonella prevalence in egg surface swab from
the open market was higher than the level of Salmonella in egg
internal contents. However there was no significant difference
of Salmonella isolation rate between surface swab and internal
contents (P value; 0.702) (Table 2). The prevalence of Salmonella
samples taken from internal contents in current study were 1.91%
which was lower than study done in Addis Ababa by Bayu et al. [31]
reported 4.7%. In farm eggs, we recorded an overall isolation rate
of 2.6 %.Various contamination levels were reported by numerous
studies in Ethiopia 2.5% from chicken eggs in Kombolcha city
Assefa et al. [32]; 2.9% in Haremaya on chicken of different breeds
Kassahun et al. [29]2.9% and 3.3% in large farms from Grenada,
West Indies (Sabarinath et al. [33]). Conversely, the present finding
was higher than Kemal et al. [29] in Haremaya that was not isolated
and lower than study done in Long et al. [34] were 33.3%.
Salmonella prevalence from eggs in this study might be
explained by environmental contamination during storage and
transportation and ineffective cleaning of eggs. Moreover, the
management system in practice could also be the probable reason
for the variation of the prevalence. Egg contamination can occur in
the infected ovary of the hen (Hossain et al. [35]) and also through
egg contact with fecal material or it might be due to contamination
of eggs during their supply from chicken owners to wholesale and
retail markets.
Antimicrobial susceptibility testing
For the antimicrobials tested, all seven Salmonella isolates were resistant to ampicillin (AMP) and tetracycline (TET). In addition, 85.7 % of isolates were resistant to cephalothin (CEP), streptomycin (S) and rifampicin (R). On the other hand, all isolates were sensitive to gentamycin, 85.7 % of isolates were susceptible to ciprofloxacin and 57.1 % were susceptible to kanamycin (K), nalidixic acid (NA) and chloramphenicol (C) (Table 3). This finding was consistent with a study conducted in Harmaya, Ethiopia by Kemal et al. [24] who reported that all isolates were sensitive to ciprofloxacin and over 70 % isolates were sensitive to chloramphenicol(C). In the current study, all Salmonella isolates develop multiple drug resistance. Extensive and inappropriate use of antibiotic drugs in food animal agriculture can establish reservoirs of antimicrobial resistant bacteria, greatly affecting public health Afema et al. [13].
Table 3: Mono drug susceptibility profile of all isolates to the respective Antimicrobials.

AMP= Ampicillin, TET= Tetracycline, CEP= Cephalothin, S= Streptomycin, K= Kanamycin, NA= Nalidixic Acid, C= chloramphenicol, R= Rifampicin, GEN= Gentamycin, CIP= Ciprofloxacin.
In current study resistance to ampicillin and tetracycline becomes hundred percent which is higher than reported 72.7 % in Haremaya by Kassahun et al. [29] and 73 % in Germany (Miko et al. [36]). The finding of this result shows that resistance pattern of isolates to ampicillin and tetracycline were in consistent with 93.1% reported in Nigeria from Salmonella isolates in chicken eggs by (Ekundayo and Ezeoke, [37]). Isolates sensitivity to Streptomycin 14.3 % in current study were lower than 100 % and Kanamycin 57 % sensitivity also lower than 91% reported by (Kassahun et al., [29]). When bacterial isolate develop resistance to more than three antibiotics; it is said to be multidrug resistant (Sweeney et al. [38]). Accordingly all the 7 isolates were developed multi drug resistant. As shown in Table 4 of the isolates were multi drug resistant to at least for 5 different antimicrobials used. In addition, only one isolate was multi drug resistant for 6 and the other one isolate for 7 antimicrobials used in this study. Multiple drug resistance was observed in over 30 % of Salmonella isolates, which is lower than the current study (100%) of isolates were developed multidrug resistant for tested antimicrobial disks. A high proportion of Salmonella isolates were developed to resist commonly prescribed antimicrobials. This may be a considerable risk in the treatment of clinical cases Addis et al. [39]. In contrast to the current study, Sibhat et al. [15] founds 18 (20.7%) Salmonella isolates were resistant more than three antimicrobials, which is lower than the current study [40-42].
Table 4: Multi-drug resistance pattern of Salmonella isolates from table eggs in Bahir Dar, 2018/19.

AMP= Ampicillin, TET= Tetracycline, CEP= Cephalothin, S= Streptomycin, K= Kanamycin, NA= Nalidixic Acid, C= chloramphenicol, R= Rifampicin, GEN= Gentamycin, CIP= Ciprofloxacin
Limitation of This Pepper
Serotyping and molecular identification of resistance Salmonella strains, as well as analyses of its antimicrobial resistance genes were not done.
Conclusion and Recommendations
The isolation rate was low in the current study. However,
more egg contamination was observed by Salmonella isolates
in eggs obtained from the farm than the market. External egg
contamination was more frequently observed than the internal egg
content. All Salmonella positive isolates were tested by ten selected
antibiotic disks for susceptibility test. A hundred percent of the
isolates were become resistant to more than five antibiotic disks.
All isolates were developed to resist ampicillin and tetracycline,
but all of them become sensitive to gentamycin. This indicates
the importance of avoiding the use of antimicrobials in growth
promoters and feed additives to reduce drug resistance. Strict
biosecurity measures in poultry farms should be encouraged to
reduce salmonella contamination of eggs. There is a need for further
study on molecular characterization and serotyping of Salmonella
strain found in table egg that is resistance.
Ethics approval and consent to participate.
References
- FAO, 2018. Global Forum on Food Security and Nutrition. Eggs: harnessing their power for the fight against hunger and malnutrition Report of activity No 154: 1-35.
- Réhault-GodbertS, Nicolas Guyot, Yves Nys (2019) The golden egg: nutritional value, bioactivities, and emerging benefits for human health. Nutrients 11(3): 684.
- Abeyrathne HY, Lee, Ahn DU (2013) Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents--A review. Poultry Science 92(12): 3292-3299.
- Galiş AM, Marcq C, Marlier D, Portetelle D, Van I, et al. (2013) Control of Salmonella contamination of shell eggs-Preharvest and postharvest methods: A review. Comprehensive Reviews in Food Science and Food Safety 12(2): 155-182.
- US Food, Drug Administration (2018) Egg safety; U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition’s.
- Whiley H, Ross K (2015) Salmonella and Eggs: From Production to Plate. Int J Environ Res Public Health 12(3): 2543-2556.
- Mei Long , Hua Yu , Li Chen , Guoyan Wu , Siyue Zhao, et al. (2017) Recovery of Salmonella isolated from eggs and the commercial layer farms. Gut Pathog 9: 74.
- Alghoribi MF, Doumith M, Alrodayyan M, AL Zayer M, Köster WL (2019) Enteritidis and S. Typhimurium Harboring SPI-1 and SPI-2 Are the Predominant Serotypes Associated with Human Salmonellosis in Saudi Arabia. Front Cell Infect Microbiol 9: 1-12.
- Jajere SM (2019) A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance, Veterinary World 12(4): 504-521.
- Ayukekbong JA, Ntemgwa, M, Atabe, AN (2017) The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control 6: 47.
- Munda MD, Khanb UH, Tahir U, Mustafab BE, Fayyaz A (2017) Antimicrobial drug residues in poultry products and implications on public health: A review. Taylor & Francis Group, LLC. International Journal of Food Properties 20(7): 1433-1446.
- Smith, DL, Dushoff J, Morris JR, JG (2005) Agricultural antibiotics and human health. PLoS Medicine 2:
- Afema J, Mather, A, Sischo W (2015) Antimicrobial Resistance Profiles and Diversity in Salmonella from Humans and Cattle, 2004-2011. Zoonoses and public health 62: 506-517.
- Beyene G, Nair S, Asrat D, Mengistu Y, Engers H, et al. (2011) Multidrug resistant Salmonella Concord is a major cause of salmonellosis in children in Ethiopia. The Journal of Infection in Developing Countries 5(1): 23-33.
- Sibhat B, Molla Zewde B, Zerihun A, Muckle A, Cole L, et al. (2011) Salmonella serovars and antimicrobial resistance profiles in beef cattle, slaughterhouse personnel and slaughterhouse environment in Ethiopia. Zoonoses and public health 58: 102-109.
- Dagnew M, Tiruneh M, Moges F, Gizachew M (2013) Bacterial profile and antimicrobial susceptibility pattern among food handlers at Gondar University Cafeteria, Northwest Ethiopia. Journal of Infectious Diseases and Therapy 1: 105.
- Abebe G (2017) Long-term climate data description in Ethiopia. Data in brief 14: 371-392.
- CSA (2013) Central Statistical Authority Statistical abstract. Addis Ababa: Federal Democratic Republic of Ethiopia.
- Abebu T (2018) RE: The Number of Farms and Population of Laying Hen in Bahir Dar Town.
- Thrusfield M (2018) Veterinary epidemiology. (3rd edn), Blackwell Science Ltd, pp. 233.
- Suresh T, Hatha A, Sreenivasan D, Sangeetha N, Lashmanaperumalsamy P (2006) Prevalence and antimicrobial resistance of Salmonella enteritidis and other Salmonella in the eggs and egg-storing trays from retails markets of Coimbatore, South India. Food microbiology 23: 294-299.
- Mooijman KA, Pielaat A, Kuijpers AFA (2019) Validation of EN ISO 6579-1 - Microbiology of the food chain - Horizontal method for the detection, enumeration and serotyping of Salmonella - Part 1 detection of Salmonella spp. Int J Food Microbiol 288: 3-12.
- Romney M Humphries, Jane Ambler , Stephanie L Mitchell , Mariana Castanheira , Tanis Dingle, et al. (2018) CLSI Methods Development and Standardization Working Group Best Practices for Evaluation of Antimicrobial Susceptibility Tests. J Clin Microbiol. 56(4): e01934-17.
- Kemal J, Sibhat B, Menkir S, Beyene D (2016) Prevalence, assessment, and antimicrobial resistance patterns of Salmonella from raw chicken eggs in Haramaya, Ethiopia. The Journal of Infection in Developing Countries, 10: 1230-1235.
- Authority EF S (2014) Multi‐country outbreak of Salmonella Enteritidis infections associated with consumption of eggs from Germany. EFSA Supporting Publications 11: 646E.
- Murchie L, Whyte P, B Horrigan, Kelly S, Madden RH (2007) Prevalence of Salmonella in grade A whole shell eggs in the island of Ireland. Journal of food protection 70:1238-1240.
- Tsegaye S, Beyene W, Tesfaye B, Tesfaye S, Feleke A (2016) Prevalence and antimicrobial susceptibility pattern of Salmonella species from exotic chicken eggs in Alage, Ziway and Shashemene, Ethiopia. Africa Journal of Basic and Applied Science 8: 180-184.
- Ejo M, Garedew L, Alebachew Z, Worku W (2016) Prevalence and antimicrobial resistance of Salmonella isolated from animal-origin food items in Gondar, Ethiopia. BioMed research international.
- Kassahun T, Hussen B, Mebrat E, Adem H (2017) Prevalence and Antibiotic Resistance of Salmonella Species Isolated from Chicken Eggs by standard Bacteriological Method. Journal of veterinary science and Technology 8: 421.
- Nguse D, Kumar A, Balcha E (2015) Prevalence of Salmonella in Eggs Collected from Local Markets and Poultry Farms in Mekelle Ethiopia. European Journal of Biological Sciences 7: 41-44.
- Bayu Z, Asrade B, Kebede N, Sisay Z, Bayu Y (2013) Identification and characterization of Salmonella species in whole egg purchased from local markets in Addis Ababa, Ethiopia. Journal of Veterinary Medicine and Animal Health 5: 133-137.
- Assefa M, Teklu A, Negussie H (2011) The prevalence and public health importance of Salmonella from chicken table eggs, Ethiopia. American-Eurasian Journal of Agriculture and Environmental Science, 11: 512-518.
- Sabarinath A, Guillaume V, Guillaume B, Mathew V, Deallie, et al. (2009) Bacterial contamination of commercial chicken eggs in Grenada, West Indies. West Indian Vet J 9: 4-7.
- Long M, Lai H, Deng W, Zhou K, Li B, et al. (2016) Disinfectant susceptibility of different Salmonella serotypes isolated from chicken and egg production chains. Journal of applied microbiology 121: 672-681.
- Hossain M, Chowdhury E, Islam M, Haider M, Hossain M (2006) Avian salmonella infection: isolation and identification of organisms and histopathological study. Bangladesh Journal of Veterinary Medicine 4(1): 7-12.
- Miko A, Pries K, Schroeter A, Helmuth R (2005) Molecular mechanisms of resistance in multidrug-resistant serovars of Salmonella enterica isolated from foods in Germany. Journal of Antimicrobial Chemotherapy 56: 1025-1033.
- Ekundayo E, Ezeoke J (2011) Prevalence and antibiotic sensitivity profile of Salmonella species in eggs from poultry farms in Umudike, Abia State. Journal of Animal and Veterinary Advances 10(2): 206-209.
- Sweeney MT, Lubbers BV, Schwarz S, Watts JL (2018) Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. Oxford University Press; J Antimicrob Chemother 73: 1460-1463.
- Addis Z, Kebede N, Sisay Z, Alemayehu H, Wubetie A, et al. (2011) Prevalence and antimicrobial resistance of Salmonella isolated from lactating cows and in contact humans in dairy farms of Addis Ababa: a cross sectional study. BMC infectious diseases 11: 222.
- Bell C, Kyriakides A (2009) Salmonella; Foodborne Pathogens; Hazards, Risk Analysis and Control. 2nd edition; Wood head publishing Series in Food Science, Technology and Nutrition. pp. 627-674.
- Kretser A, Dunn C, Devirgiliis R, Levine K (2014) Utility of a new food value analysis application to evaluate trade-offs when making food selections. Nutrition Today 49: 185-95.
- Moffatt CR, Appuhamy R, Kaye A, Carswell A, Denehy D (2012) An outbreak of Salmonella Typhimurium phage type 135a gastroenteritis linked to eggs served at an Australian Capital Territory café. Communicable diseases intelligence quarterly report 36(3): E281-E285.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...





