Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4749
Review Article(ISSN: 2637-4749) 
Methane Production in Ruminant Animals: Implication for Their Impact on Climate Change Volume 2 - Issue 4
Mebrate Getabalew1, Tewodros Alemneh2* and Dawit Akeberegn3
- 1College of Agricultural and Natural Resource Science, Department of Animal Science, Debre Berhan University, Ethiopia
- 2Woreta Town Office of Agriculture and Environmental Protection, South Gondar Zone, Amhara Regional State, Ethiopia
- 3Debre Berhan City Municipality Office, Meat Inspection and Hygiene, Amhara Regional State, Ethiopia
Received: January 03, 2019; Published: April 04, 2019
Corresponding author: Tewodros Alemneh, Woreta Town Office of Agriculture and Environmental Protection, South Gondar Zone, Amhara Regional State, Ethiopia
DOI: 10.32474/CDVS.2019.02.000142
Abstract
Agriculture accounts for about 47%-56% of the total anthropogenic methane (CH4) emission. It is known that from the agricultural sector, ruminant livestock (dairy, beef, goats, and sheep) substantially contributes to the increase in CH4 production through continuous natural rumen fermentation process. Methane emission is now the second contributor to global warming, which it has 23 times more influence than that of carbon dioxide (CO2). Many factors affect the amount of ruminant CH4 production, including level of feed intake, type and quality of feeds, energy consumption, animal size, growth rate, level of production, and environmental temperature. Methane also produced from manure of animals depending on the physical form of the faeces, the amount of digestible material, the climate, and the time they remained intact. The major part of methanogenesis in ruminants occurs in the large fermentative chamber, which is rumen. Ruminal digestion of feed by microorganisms, under anaerobic conditions, results in the production of acetate, propionate and butyrate (volatile fatty acids) which are used by the animal as energy source, and production of ruminal gases such as carbon dioxide (CO2) and CH4, which eliminated through eructation. Therefore, the aim of this review was to summarize the current status of methane production from ruminants and its implication for their impact on climate changes.
Keywords: Emissions; Global Warming; Manure; Methane; Microorganisms; Rumen; Ruminants
Introduction
Ruminant livestock are the primary producers of methane (CH4). They can produce 250 to 500 liters of methane per day [1]. This level of production results in estimates of the contribution by cattle to global warming is high. Many factors influence these methane emissions from cattle. This includes like, level of feed intake, type of carbohydrate in the diet, feed processing, etc. Manipulation of these factors can reduce methane emissions from cattle. Ruminal digestion of feed by the microorganisms, under anaerobic results in the production of acetate, propionate and butyrate, which are used by the animal as energy source, and the production of carbon dioxide ( CO2) and CH4 which eliminated through eructation [2]. This all gases are produced in the rumen by the process of methanogenesis. It is a process besides its negative impact on the environment, representing a loss of 2-15% of gross energy intake [3] for the animal, leading to an unproductive use of dietary energy [4]. Techniques to manipulate this process include elimination of protozoa [5], use of antibiotics (like Monensin and bacteriocins such as Nisin) [6], use of lipid sources [7], organic acids [8] and ionospheres [9] or change in dietary composition [10]. In the global worming view, CH4 is particularly the major greenhouse gas (GHG) which has a global potential 23 times that of carbon dioxide [11], and accounts for 16% of the total global GHGs emissions. From livestock, most CH4 is produced from enteric fermentation, which is a natural process produced by ruminant animals, being responsible for one-third of methane from agriculture [12]. The enteric methane produced by ruminants has its origin in the rumen [2]. Globally, atmospheric CH4 concentration increased between pre-industrial times and 2005 from approximately 0.715ppm to 1.774ppm [13]. The world atmospheric load of CH4 was 4850 Megaton in 1998 year, equivalent to an average concentration of 1745ppb [14]. The concentration of CH4 in the atmosphere is thought to be increasing at a rate of 22 Megaton per year, due to the imbalance between estimated annual global emissions of 598 Megaton and removals of 576 Megaton [15]. Hence, the objective of this review is therefore to summarize the current status of CH4 production from ruminants, and its influence on global warming.
Over View of Methane
General Characteristics
Methane is one of the three main greenhouse gases, together with CO2 and nitrous oxide (N2O). Its global warming potential is 23-fold than that of CO2 [11]. Methane is a colorless, odorless, inflammable, and tasteless gas that is the primary component of natural gas. Methane is present naturally in the atmosphere; CH4 is lighter than air and has a specific gravity of 0.554. Methane gas has a density of 0.717m-3/kg, melting point of -187 °C, and a boiling point of -161°C. This gas is only not soluble in water but is soluble in organic solvents. Naturally occurring CH4 is mainly produced by the process of methanogenesis. The reaction is: CO2 + 4H2 → CH4 + 2H2O [16].
Source of Methane from Ruminants
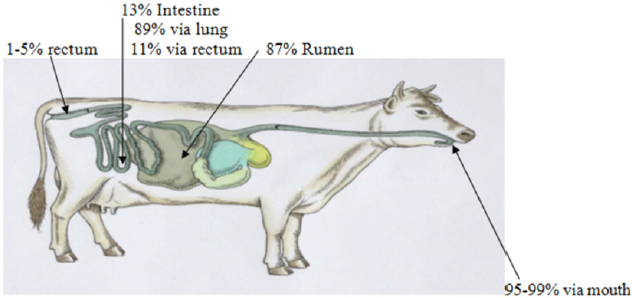
Methane from Enteric Fermentation: According to Dawo [1], enteric CH4 is a by-product of ruminant digestion produced by methanogenic microorganisms, Archaea, by the process called fermentation or methanogenesis. The rate and type of fermentation is influenced by animal factors such as chewing, salivation and digesta kinetics [17]. Cattle produce about 7 and 9 times as much CH4 as sheep and goats, respectively. Enteric CH4 is produced mainly in the rumen (87% - 90%) and, to a lesser extent (13% - 10%), in the large intestine [18]. Animal releases CH4 into the atmosphere by exhaling the gas mainly through the mouth and nostrils [13]. Of the CH4 produced by enteric fermentation in the fore stomach, 95% was excreted by eructation, and from CH4 produced in the hindgut, 89% was found to be excreted through the breath, and only 11% released through the anus [19] (Figure 1). Work by Munoz et al. [20] recorded 3% from the anus (from the total CH4 enteric emissions released through mouth, nostrils, and rectum). The concentration in the breath is variable with a relatively low concentration when the breath comes from the lungs and a higher concentration when the “breath” is gases belched from the fore stomachs although breath from lungs also contain absorbed CH4 and inhaled together with air [21]. In a barn or larger room, the concentration will to a large extent be influenced by the air exchange, but the concentration of CH4 will be a total mix of the CH4 from breath, belch and fart [21].
Methane from Manure: In addition to enteric CH4 production, excreta are another source of CH4, especially when stored an aerobically [22]. Methane generated from manure from ruminant and no ruminant livestock contributes 2% and 0.4% of global CH4 and GHG emissions, respectively, in regions with low input is enteric fermentation undoubtedly the main emission source. However, in industrialized regions with high production and food processing is important source of emissions [23]. Manure CH4 emissions are a larger proportion of total farm CH4 emissions in intensively managed dairy operations with manure storage systems, and much lower in extensive or Grazing operations because it is mostly aerobic condition [24]. Livestock manure contains portion of organic solids such as proteins, carbohydrates and fats that are available as food and energy for growth of anaerobic bacteria [1]. Obviously, benefit from methane production could be the energy value of the gas itself, but the gas production from manure depends mainly upon the efficiency of operating system for it. Gas yield can be a certain amount of gas produced per unit of solids degraded by the anaerobic bacteria [25]. Anaerobic digestion is a natural process in which the microorganisms consume organic matter under an oxygen-free environment [1]. It results in production of microbial biomass and GHG (CO2 and CH4). The composition of volatile solids contained in manure influence the anaerobic decomposition of organic matter and the production of CH4. The manure volatile solids are mainly composed of fatty acids, proteins and carbohydrates of which fatty acids, proteins and a part of carbohydrates are easily biodegradable [26].
Methanogens and Ruminal Methanogenesis
Methanogens: Methanogens are a distinct group of microorganisms [27] which belong to the domain Archaea and the phylum Euryarchaeota [16]. Among methanogens, the cell shape and characteristics vary as well. The most important methanogen found in rumen, is Methanobrevibacter ruminantium, with pseudo murein in the cell envelope and requires coenzyme [27] hydrogen, carbon dioxide and formate for methane production [28]. Methanogens species have been classified into 28 genera and 113 species, but in the nature can be expected to occur many more [29]. From the rumen, few methanogens have been isolated. The cultured methanogens have been assigned to seven species: Methanobrevibacter ruminantium, Methanobrevibacter millerae, Methanobrevibacter olleyae, Methanobacterium formicicum, Methanobacterium bryantii, Methnaomicrobium mobile and Methanoculleus olentangyi [29].
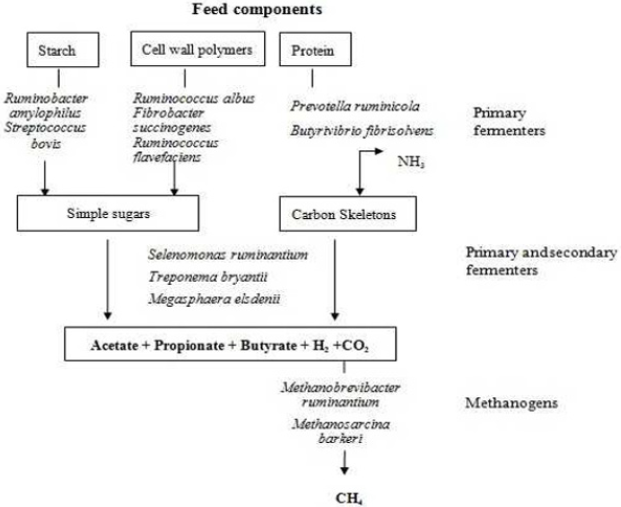
Ruminal Methanogenesis: The major part of methanogenesis in ruminants occurs in the large fermentative chamber known as rumen [30]. In here, methanogens utilize hydrogen and CO2 to produce CH4 (Figure 2).
Feeding Effects on Methane Production
Ramin and Huhtanen [31] concluded that feed intake is the superior factor for total CH4 production from ruminant animals. The amount of enteric CH4 is mainly related to the type and amount of feed [32]. Gross energy (GE) is negatively related to feeding level and dietary fat concentration, and positively related to diet digestibility, whereas dietary carbohydrate composition has only minor effects. Generally, as the daily feed intake increases, CH4 production also increases [32]. Most studies agree that dry matter intake (DMI) is the main driver of daily methane output although methane output per kilogram of DMI decreases with increasing feeding level [33], diet digestibility, and with increasing proportions of concentrates or lipids in the diet [34]. The composition of feed or the quality of forage influences CH4 production in ruminants. Digestion in the rumen is dependent on the activity of microorganisms, which need energy, nitrogen and minerals [32]. Therefore, the quality of forage affects the activity of rumen microbes and CH4 production in the rumen. Forage species, forage processing, proportion of forage in the diet, and the source of the grain also influence CH4 production in ruminants. Methane production tends to decrease as the protein content of feed increase and increases as the fiber content of feed increases [32]. Methane production is positively related to diet digestibility and negatively related to dietary fat concentration, whereas dietary carbohydrate composition had only minor effects [35]. Production of CH4 has a negative impact on animal productivity, resulting in lost energy ranging from 2% to 12% of the animal’s GEI [36].
Amount of Methane Produced
The atmospheric air contains a small concentration of CH4 (1.8ppm), compared to respiration air of cattle, approximately 1000ppm [21]. The barn concentration of CH4 is a mixture of air from background (outdoor concentration representing atmospheric air) and gases excreted from the animal. Moreover; if manure is kept in the animal barn, some CH4 may originate from this manure [21]. Methane production is measured by different methods [37] in absolute as well as relative units. For instance, it can be measured by using the ratio of emissions to the live weight (LW), per unit of feed intake (DMI), or fat and protein corrected milk (FPCM).
Dairy Cattle: Methane emissions by dairy cows vary with body weight, feed intake, diet composition, and milk yield. When cows are fed the same diet at the same intake, however, variation between cows in CH4 emissions can be substantial [38]. Primary sources of CH4 on dairy farms are not only animals but also manure storage, with smaller contributions from field-applied manure, feces deposited by grazing animals, and manure on barn floors. Chianese et al. [35] calculated in simulating representative 100- cow dairy farm annual emissions of 142kg CH4 per Holstein cow and 6.4kg CH4 per of slurry manure in storage. Feed intake was the primary predictor of total CH4 production.
Beef Cattle: Laubach et al. [39] showed that steers of average LW 325kg based on the animal-scale method, the average CH4 emission rate over 9 days was 161±20g/day. There was a significant difference between two contrasting diets (Lucerne silage diet, cereal, Lucerne, and straw mixed ration) in daily CH4 production, with mean methane production of 124.3g/day and 169.8g/day [40]). On average, mature beef cows emit CH4 from 240g/day to 350g/day [41]. Huarte et al. [42] found that the CH4 emission rates corresponding to values of 190g/day per beef cattle head, and [43-46] recorded annually 60kg/head. The results of McGinn et al. [44] show that daily CH4 emissions differed about 7% according to different techniques used (185 vs. 199g·day−1 per animal). Animals kept in feedlots, as opposed to pasture; emit less CH4 per Kg of weight gain due to decreased forage consumption, increased grain in the diet and decreased activity [36].
Methods of Methane Reduction
Reductions in methane production from ruminant animals can result from a reduction in rumen fermentation rate (suppression in microbial activity) or a shift in volatile fatty acid (VFA) production [45]. An inverse relationship exists between the production of CH4 in the rumen and the presence of propionate [1]. If the ratio of acetate to propionate was greater than 0.5, hydrogen would become available to form CH4 [46]. If the hydrogen produced is not correctly used by methanogens, such as when large amounts of fermentable carbohydrate are fed, ethanol or lactate can form, which inhibits microbial growth, forage digestion, and any further production of VFAs. In practice, ethanol or lactate may form, but any excess hydrogen is simply eructated [19].
Uses of Ionophore Antibiotics: The most effective antibiotic in ruminant fermentation is monensin, although others such asnigercin, gramicidin and lasalocid are available [47]. Monensin is produced by Streptomyces cinnamonensis and it is known to increase milk production [48]. These drugs do not alter the diversity and quantity of rumen methanogens [16]. They only shift the bacterial population from gram-positive to gram-negative and this means a change in rumen fermentation from acetate to propionate [30]. This is the reason why monensin does not affect methane production by altering the methanogens population, but instead, inhibits the growth of bacteria and protozoa [16].
Plant Extracts as Feed Additives: Some feed additives from plant extracts have been analyzed for their ability to reduce rumen CH4 production [49]. Such plant extracts are saponins, tannins and essential oils, but in the last years many other feed additives were studied. Tavendale and Coworkers [50] used condensed tannins from Lespedeza cuneata against rumen CH4 production and found that reduced methane emissions by up to 57% in terms of g/kg DMI. Other authors found that sheep consuming 41g of tannin containing Acacia mearnsii per kg DM produced methane with 13% less than sheep feed normal forage [51]. Saponins containing Sapindussaponaria reduced methane emissions by up to 20%without affecting methanogens number [52]. In other studies, saponins were found to inhibit protozoa number in vitro and to limit hydrogen availability for methanogenesis [3]. It was found that essential oils present the same effect such as monensin by inhibiting gram-positive bacteria [16].
Uses of Lipids as Feed Additives: Lipids are an option for feed supplementation that has been studied for their effects on methanogenesis process [16]. Oils, such as coconut oil, was used in simulators against rumen fermentation and showed that the main component (lauric acid) inhibited methanogenesis [53]. But it has negative effect on digestibility of feed so not feed lipid more than 10% to the animal.
Defaunation: Defaunation represents the process that eliminates protozoa population from the rumen because methanogens attached on protozoa [1]. This treatment has been used to investigate the role of protozoa population in rumen and to study the effect on methane production [16]. It is known that methanogens in rumen are attached on protozoa and they share a symbiotic relationship with participation in hydrogen transfer [54]. Methanogens species that are associated with protozoa are responsible for 9 to37 % of the methane production in the rumen [54] and for this reason treatments that affect protozoa population in rumen may have an effect on methanogenesis process [16]. Hegarty et al. [5] suggested that defaunation treatment reduced methane with 13% but this impact varied with the diet. Other authors suggested that defaunation had an effect on rumen methanogens for more than two years and diet supplementation with ionophore reduce methane production in short-term [5].
Immunization Against Rumen Methanogens: In the last years, researchers tried to found a way to inhibit methanogens actions without affecting other ruminal microorganisms. For this, it is essential to evaluate methanogens-specific targets. The genome sequence of Methanobrevibacter ruminantium, strain M1 by Leahy et al. [49] provided new perspective on the life style of the most important methanogen found in rumen. This is also essential in evaluation of a vaccine against methanogenesis process, which can be a long-term methane mitigation technology. In addition, there are many strategies to reduce the production of methane from the rumen of ruminant animals, like proper feeding of animal, reduce movement of animal etc.
Factor that Affect Methane Production
Diet Type
The type of feed allowed to a ruminant can have a major effect on methane production. Forage to concentrate ratio of the ration has an impact on the rumen fermentation and hence the acetate to propionate ratio [1]. It would therefore be expected that methane production would be less when high concentrate diets are fed [55]. According to Van Soest [56], a high grain diet and/or the addition of soluble carbohydrates gave as shift in fermentation pattern in the rumen which give rise to a more hostile environment for the methanogenic bacteria in which passage rates are increased, ruminal pH is lowered and certain populations of protozoa, ruminal ciliates and methanogenic bacteria may be eliminated or inhibited. The work of Lana et al. [57] supports this theory confirming that low rumen pH regulates CH4 production.
Forage Type and Supplementation
Supplementing forages whether of low or high quality, with energy and protein supplements, is well documented to increase microbial growth efficiency and digestibility [12]. Milk and meat production will increase as a result. The direct effect on methanogenesis is still variable and unclear, but indirectly, methane production per unit product will decline. Increasing the level of non-structural carbohydrate in the diet (by 25%) would reduce CH4 production by as much as 20%, but this may result in other detrimental effects including acidosis, laminitis and fertility problems [1]. In addition, many other factors which affect CH4 production like season, age of animal, management of animal, and population of protozoa in the rumen.
Methods of Methane Measurement
Respiration chamber
According to Dawo [1], principle of the chamber is to collect exhaled CH4 emissions from all sources of enteric fermentation (mouth, nostrils, and rectum) from the animal and to measure the concentration methane. Chambers are divided into two types, the closed-circuit and the open-circuit. The closed-circuit system is almost not used and preferred are open-circuit chambers [28,58]. An air pump removes all air from the space through a flow meter and gas sensors in the open-circuit system. Each chamber is fitted with internal ventilation fans for efficient mixing of expired gases and incoming air [1]. Air inlet is located at the front and an air outlet at the back. Fresh air to chamber is directly drawn from outside or through an air conditioning system to control humidity and temperature. The chamber is equipped with sensors for measuring relative humidity, temperature and pressure. These allow air flow data to be adjusted for dry, standard temperature and pressure conditions. Outlet gas from each chamber is continuously sampled for analysis. Air flow is ducted via flexible polyurethane hoses. Air circulation is provided throughout the chambers at continuous but adjustable flow rates (usually 100-250L/m) [28,58] (Figure 3).
Proxy Methods
This method was developed with the purpose of examining many animals at the same time without complex and expensive equipment. Close relationship of methane emissions with parameters that can be measured in easily obtainable from samples of milk or feces is used [59]. Usually, the fatty acid profiles of milk are examined for correlations with CH4 production of the cows. The principle is that some fatty acids or fats in the milk or feces are correlated with either the feed composition or the amount of methanogens in the rumen [60,61].
In Vitro Gas Method
The gas measuring technique has been widely used for evaluation of nutritive value of feeds. More recently, the increased interest in the efficient utilization of roughage diets has led to an increase in the use of this technique due to the advantage in studying fermentation kinetics; gas measurement provides a useful data on digestion kinetics of both soluble and insoluble fractions of feedstuffs [62]. According to Navarro-Villa et al. [63] and Storm et al. [58], the principle is to ferment feed under controlled laboratory conditions by natural rumen microbes. Feedstuffs are incubated at 39°C with a mixture of rumen fluid, buffer and minerals for a certain time period. The amount of total gas produced during incubation is measured and its composition analyzed, to obtain data on the in vitro production of CH4. This method requires access to fresh rumen fluid, which is typically obtained from fistulated cows or slaughter of other ruminant animals.
In Vitro Gas Method
Alternative Methods
More applications of alternative methods are combined with milking and feeding [64]. The animals entering in automatic milking or feeding system are recognized and concentrations of CH4 and CO2 are measured. Air is continuously pumped through the equipment to quantify flow and there by CH4 and CO2 emitted during milking and feeding. Garnsworthy et al. [64] developed a novel technique based on sampling air released by eructation during milking. Methane analyzers are installed in automatic milking stations. Belching frequency and methane released per eructation are used to estimate CH4 emission rate.
Conclusion
From livestock, ruminants are the primary producers of CH4. They can produce 250 to 500 liters of methane per day. In general, management of animal is an important contributor to global emissions of greenhouse gases, in particular of CH4. This review summarized the current state of knowledge on methane production relevant to environmental aspects. Reduction of ruminal methane production in ruminants is a difficult issue. However, we can achieve progress towards reducing methane production from biotechnologies, reducing the number by increasing the efficiency of animals, producing high quality forages and pastures, use of alternative forage and concentrate feeds which has high content of ingredients such as tannin and saponin, and also using of probiotics.
Acknowledgements
Authors’ deepest gratitude forwarded to those who had participated and supported this work to become available worldwide for the entire clock to readers and investigators.
References

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...