
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4749
Opinion(ISSN: 2637-4749) 
Is the Water Really Safe to Drink? A Potential Threat of Cyanotoxin Poisoning of Livestock by Water Supplies to Facilities Cyanotoxin Poisoning of Livestock in Facilities Volume 3 - Issue 2
Jian Yuan1,2*
- 1Department of Veterinary Diagnostic and Production Animal Medicine, Iowa State University, USA
- 2Veterinary Diagnostic Laboratory, Iowa State University, USA
Received: January 14, 2020; Published: January 21, 2020
Corresponding author: Jian Yuan, Department of Veterinary Diagnostic and Production Animal Medicine, Iowa State University, USA
DOI: 10.32474/CDVS.2020.03.000160
Abstract
Freshwater toxic cyanobacteria can produce potent cyanotoxins which are detrimental and lethal to animals. Water from farm ponds contaminated by cyanotoxins can carry the toxins to nearby livestock facilities, posing a threat of animal poisoning when toxins reach a dangerous level. Therefore, fast and precise means should be conducted to reveal the existence and levels of cyanotoxins and toxic cyanobacteria to find out such threats in a timely fashion.
Keywords: Toxic cyanobacteria; cyanotoxins; farm ponds; livestock; facilities
Abbreviations: HAB: Harmful Algal Bloom; ELISA: Enzyme Linked Immunosorbent Assay; HPLC: High Performance Liquid Chromatography; MS: Mass Spectrometry; PCR: Polymerase Chain Reaction
Introduction
Cyanobacteria, colloquially called blue-green algae, are a group
of photoautotrophic prokaryotic microalgae which inhabit a variety
of environments on earth. Their ubiquity in global freshwater
bodies has made them important cosmopolitan organisms in
freshwater habitats. Being able to proliferate explosively under
appropriate ambient conditions, for example sufficient nitrogen
and/or phosphorus nutrients, cyanobacteria can easily become
dominant in inhabited ecosystems and form an ecological
phenomenon/disaster named HAB. An HAB can bring about many
deleterious impacts on local ecosystems such as hypoxia caused
by over consumption of oxygen by bacteria for degrading dead
cyanobacterial cells. What is even worse, some cyanobacteria can
produce potent cyanotoxins toxic to animals and humans, and the
toxins can be enriched to a dangerous level in an HAB, causing health
and sanitation problems [1,2]. Cyanotoxins contain a tremendous
number of species and are categorized by their toxicological
effects into hepatotoxin, neurotoxins, cytotoxin, etc. Microcystin,
anatoxin-a, and cylindrospermopsin are the most prevalent
representative toxins that fall into the three categories, respectively.
They can kill a poisoned animal in a very low dose (e.g, 3 ppb for a
nursery pig) [1]. As a secondary metabolite, these toxins are mostly
produced by cyanobacterial species in Microcystis, Anabaena, and
Cylindrospermopsis, respectively, although their production is also
found in other genera. The process of toxin production is well
profiled and regulated under a synthetic mechanism by a group
of enzymes that are translated from clustered toxin synthetase
genes [3-5]. Direct detection of cyanotoxins can be conducted using
ELISA, HPLC, and MS. These techniques are already characterized
with satisfactory specificities and sensitivities. Moreover, detection
of toxic cyanobacteria can be done as an alternative approach for
identifying toxicity. Because a toxic species actually has toxic strains
as well as non-toxic strains that share identical morphologies but
lack the toxin synthetase genes, traditional microscopic examination
cannot fulfill the purpose of accurate recognition of toxicity. As a
result, molecular methods targeting the existence of toxin genes is
an effective approach to find out the toxic cyanobacteria, of which
PCR is the most popular. While, intuitively, existence of genes doesn’t
equate to production of toxins as there is likely a “switching on/off”
mechanism of the genes (frankly, “toxigenic” is more accurate than
“toxic” per se), findings of good correlations for their existence as well as quantities suggest revelation of toxic cyanobacteria is an
excellent indicator of toxicity [6].
Microcystis and Anabaena exist across extensive latitudes, and
Cylindrospermopsis can be discovered in temperate zones despite
it was found in tropical zones. They grow in some waterbodies
which are also water sources for livestock. Although the water is
sometimes treated to remove possible contaminants, it is directly
transported to facilities for animals’ consumption, increasing the
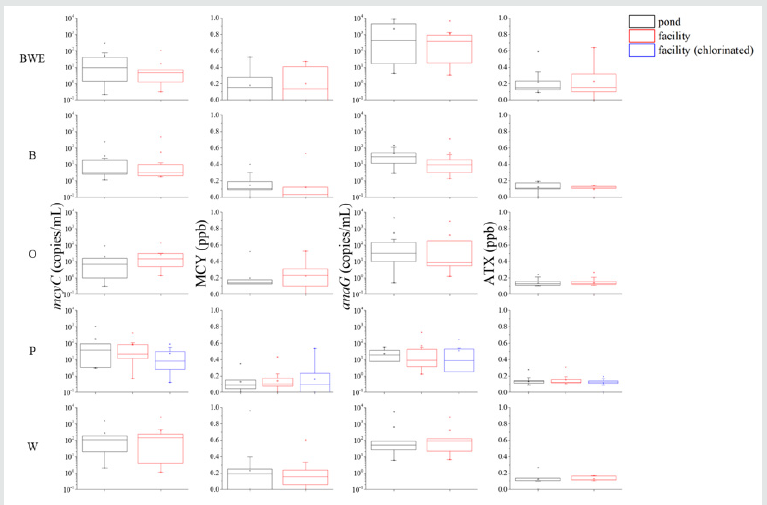
likelihood of cyanotoxin poisoning. In my recent study in five farm
ponds and five swine facilities served by these ponds as water
sources in the Midwestern United States, it was found that the
abundances of toxic Microcystis spp. and Anabaena spp., and levels
of microcystin and anatoxin-a had no significant differences (p >
0.05 by paired t-tests) in water between ponds and facilities with
a few chlorinated samples in one facility for detoxification (Figure
1). Although all the quantities were far below the warning levels for
an HAB or a toxicosis, chronical effects may occur on swine due to
accumulation of toxic cyanobacteria and/or toxins. More crucially,
swine can easily be poisoned once toxins are rapidly accumulated
in an unpredictable HAB event. Furthermore, the failure of
decontamination of cyanotoxins by chlorination suggests an option
of effective detoxification.
Figure 1: Abundances of toxic Microcystis spp. and Anabaena spp, and levels of microcystin and anatoxin-a in 105 water samples from five farm ponds and five swine facilities in the Midwestern United States. Toxic Microcystis spp. and Anabaena spp. are represented by one synthetase gene for microcystin and anatoxins-a (mcyC and anaG), respectively. MCY and ATX are abbreviations for microcystin and anatoxins-a, respectively. Ponds and facilities are indicated by BWE, B,O,P, and W. Black, red, and blue squares refer to pond water, facility unprocessed water, and chlorinated water, respectively..
Conclusion
In farm ponds as water resources to facilities lies a potential threat of livestock poisoning by cyanotoxins produced by toxic cyanobacteria. Therefore, accurate and prompt detection approaches for toxins and their producers are highly necessary. Nowadays, detection of toxic cyanobacteria and cyanotoxins is a component of routine water monitoring programs for large and important waterbodies like lakes and reservoirs. However, such a program doesn’t exist for small farm ponds that are prone to eutrophication (over enrichment of nutrients) causing HABs. As they are vital for livestock health as water sources, it is strongly suggested monitoring of toxic cyanobacteria and cyanotoxins should be set up in these farm ponds as a diagnostic or prewarning tool for veterinarians, producers, and stakeholders.
References
- Classen DM, Schwartz KJ, Madson D, Ensley SM (2017) Microcystin toxicosis in nursery pigs. Journal of Swine Health and Production 25(4): 198-205.
- J Swine He Pouria S, de Andrade A, Barbosa J, Cavalcanti RL, Barreto VTS, et al. (1998) Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet 352(9121):21-26.
- Pacheco AB, Guedes IS, Azevedo SM (2016) Is qPCR a reliable indicator of cyanotoxin risk in freshwater? Toxins 8(6): 172.
- Tillett D, Dittmann E, Erhard M, Dohren HV, Borner T, et al (2000) Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: an integrated peptide-polyketide synthetase system. Chem Biol7(10):753-64.
- Mejean A, Paci G, Gautier V, Ploux O (2014) Biosynthesis of anatoxin-a and analogues (anatoxins) in cyanobacteria. Toxicon 91:15-22.
- Mihali TK, Kellmann R, Muenchhoff J, Barrow KD, Neilan BA (2008) Characterization of the gene cluster responsible for cylindrospermopsin biosynthesis. Appl Environ Microbiol 74(3):716-722.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




