
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4749
Research Article(ISSN: 2637-4749) 
Efficacy of Fennel seed and Koseret Leaf Powders against Pulse Beetle (Callosobruchus Chinensis L.) in Stored Chickpea (Cicer Arietinum L.) Volume 3 - Issue 4
Getachew Geresu, Zekeria Yusuf* and Sewnet Mengistu
- School of Biological and Biotechnological Sciences, Haramaya University, Ethiopia
Received: February 18, 2020; Published: March 04, 2020
Corresponding author: Zekeria Yusuf, School of Biological and Biotechnological Sciences, Haramaya University, Ethiopia
DOI: 10.32474/CDVS.2020.03.000168
Abstract
Pulse beetle (Callosobruchus chinensis L.) also commonly called bruchid, is one of the most destructive and cosmopolitan pests
of stored legume. The present study was carried out to investigate the efficacy of botanical pesticides like funnel seeds and koseret
leaf in management of pulse beetle (C. chinensis) in stored chickpea. An experimental design of A 1 x 1 x 3 [200grams of chickpea
grains + 20 pulse beetles + (5gm, 10gm, and 15gms of funnel seed, koseret leaf, admixture of both funnel seed and koseret leaf
powders)] factorial experiment was laid in a Complete Randomized Design (CRD) in two replications. The result of repellence test of
pulse beetle against fennel seed and koseret leaf powders has indicated that, all the treatments have shown high repellence against
pulse beetle at all amounts of treatments (5, 10, and 15grams) across the whole durations.
Mortality of pulse beetle, C. chinensis adult at different days after treatment with fennel seed and koseret leaf powders in solo
and in their combined forms has shown significantly the highest pulse beetle mortality rate (60%) for koseret leaf powders followed
by the admixture of koseret leaf and fennel seed powders during 20th days of treatment indicating toxicity effect of these powders
on pulse beetle. The Effect of different concentrations of fennel seed and koseret leaf powders on chickpea seed damage as weight
loss and germination loss caused by pulse beetle revealed that, there was no significance difference in weight loss and germination
potential of chickpea seeds among treatments indicating that treatment with fennel seeds and koseret leaf powders did not affect
the quality of chickpea seeds. The result of the present study demonstrated that both fennel seed and koseret seed can be used in
control of pulse beetle. Further studies are required to test the effect of these botanical powders in the form of chemical extracts, or
formulations with other ecofriendly pesticides in stored grains and foliage pests.
Keywords: Ecofriendly pesticides, Germination test, Mortality rate, Repellence test, Seed quality, Weight loss
Introduction
Pulses are important food crops as they nourish mankind with highly nutritive food being rich source of high protein and several essential amino acids. Apart from being an important source of dietary protein for human being, the pulse crops are also important for the management of soil fertility through biological nitrogen fixation in soil and thus play a vital role in furthering sustainable agriculture [1]. Chickpea, Cicer arietinum(L.) is one of the major pulse crops grown. Chickpea, besides a rich source of highly digestible dietary protein (17-20%) and 52-70 per cent carbohydrate. It is also a rich source of calcium, iron, niacin, vitamin ‘C’ and vitamin ‘B’. Its leaves consist of mallic acid which is very useful for stomach ailments and blood purification. Its feed and straw are highly rich in nutrients and are mostly used as productive ration for animals. Its protein is of high quality as compared to other pulse crops[2]. Apart from that it serves as a good source of energy (416 calories/100 gm), fat (4-10%), minerals (calcium, phosphorus, iron) and vitamins. It also helps in lowering the cholesterol level[3].
The production of chickpea is greatly hampered by both biotic and abiotic stresses and while addressing the biotic stresses, insect pests of chickpea play a significant role both in the field and in storage, limiting the chickpea production and market value. Many insect pests including lesser grain borer, red flour beetle, Grainary weevil damage chickpea in storages however, pulse beetle Callosobruchus chinensis L. is the most damaging one. Being cosmopolitan, it also damages lentil, cowpea, mung, sorghum and maize[4]. It enters inside grains by making holes and start feeding until full damage. Normally infestation starts in the field because adult beetles can easily fly and lay eggs on the chickpea pods. Infestation is caused by grubs as well as adults[5]. Male and female pulse beetles can easily be distinguished on the basis of their antennae. Males have pectinate and females have serrate antennae[6]. Its female is larger than male (Howe and Currie, 1964). Normally 6-8 overlapping generations are observed in a year[7]. Mostly fumigants and synthetic insecticides are used in grain storages to manage insect pests that lead to issues like resistance, resurgence andpoisoning of food[8].
There are a considerable quantitative and qualitative losses due to insect pests especially in stocks of chickpeas. The losses are primarily due to the attacks of insect pests, particularly the Chinese beetle (Callosobruchus chinensis L.). This pest is a potentially ubiquitous cosmopolitan beetle which can infest its host plant Cicer arietinum L. both in the field and in stocks. Within the framework of plant health protection, the use of insecticides had always been the solution [9]. But use of insecticides has had bad consequences, such as increased resistance (where increasingly insatiable species have appeared), an imbalance of the ecosystem (the massive and random destruction of the harmful and useful insects), and disturbances of the environment as there is a risk of toxicity due to the problems of residues[10,11]. With this view, the present was carried out to investigate the efficacy of botanical pesticides like funnel seeds and koseret leaf in management of pulse beetle (Callosobruchus chinensis) in stored chickpea.
Material and Methods
The experiment was conducted in Biotechnology Laboratory, School of Biological Sciences and Biotechnology, Haramaya University. Four-kilogram chickpea seeds were obtained from Haramaya University Rare Research Station and pulse beetle infested chickpea grain was bought from Shewaber market, Harar city. The grain was sieved to remove dead seed, dirty and broken particles. Then, 2kg grains were randomly sampled and stored in a refrigerator for 2 weeks to kill any prior sources of the pulse beetle inoculum and eggs that might be already pre-existing in the grain as procedure followed by Parugrug & Roxas [12]. After 2 weeks in the freezer, subsamples of 200g grains were placed in 375ml bottles with perforated lids to prevent weevils from escaping and for aeration. one-kilogram Funnel seeds and koseret leaf powders will be bought from local market at Haramaya town. The funnel seeds and koseret leaves samples will be air-dried under shade at an ambient temperature to avoid photo degradation of active ingredient by ultra-violet ray and koseret leaf sample was chopped and sun dried. The dried materials were then ground into fine powder using grinding machine and sieved with a 10mm sieve. The fine powders were then kept in air-tight containers until required.
Treatment and experimental Design
A 1 x 1 x 3 [200grams of chickpea grains + 20 pulse beetles + (5gm, 10gm, and 15gms of funnel seed, koseret leaf, admixture of both funnel seed and koseret leaf powders)] factorial experiment was laid in a Complete Randomized Design (CRD) in two replications.
Preparation of Insect Culture
The parent stock of Callosobruchus chinensis was obtained from infested chickpea grains. The insects were cultured under room temperature. The food media for the insect culture was 1kg chickpea grains for C. chinensis. 500gm of chickpea food medium was weighed into two different glass jars. Two hundred adult insect pests were introduced into each culturing medium. The culturing spanned for 50 days; at the end about eighty (540) adult insect pests will be randomly selected for the study.
Test for Repellence
The method employed by Garcia [13] with some modifications
was followed. Briefly, transparent plastic tubing, 13cm long x 1.3cm
diameter as test cylinders were used in the experiment. Each test
cylinder was plugged at one end with fine mesh tulle containing 5,
10 and 15grams of funnel seed, koseret leaf, and admixture of both
powders, while the other end was plugged with clean cotton ball
which served as a control. About 20 pulse beetles were introduced
at the middle of each test cylinder through a hole at the middle
portion of the cylinder. The hole was covered with nylon tulle mesh
to keep the insects inside the cylinder. The cylinders were grouped
accordingly to represent the treatments and replications. Each
treatment consisted of three cylinders and replicated twice.
The cylinders were left undisturbed and the number of weevils
that moved towards the untreated halves of the cylinders were
counted and rated every hour for the first five hours and at 24,
48, 96 and 144 hours thereafter. Repellency rating was calculated
following the formula:
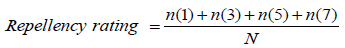
Where: n = number of insects stayed 0, 1-2, 3-4 and 5-6 cm from the center of the cylinder towards the untreated cotton plug, respectively. 1, 3, 5 and 7 = rating scale on the reaction of the insects on different test materials. N= Total number of insects introduced per cylinder. The degree of repellency of each test material was based on the following scale (Table 1).
Test for Weevil Mortality
Two hundred grams of chickpea grains adjusted to 10% moisture content (MC) was treated with 5, 10, & 15g of each of the test treatment in 12 cm high x 6.5 cm diameter glass jars. In the first treatment funnel seed powder; in the second experimental unit koseret leaf powder, and in the third experimental unit admixture of both funnel seed and koseret leaf powders were used. The admixtures were shaken manually for 5 minutes and then tumbled for 15 minutes in a mechanical tumbler. The treated grains were left undisturbed for an hour. Thereafter, mixture 20 adult pulse beetles were introduced per treatment. The glass jars were covered with filter paper and sealed with molten wax to keep the insects inside. Untreated chickpea grains were served as control. Each treatment was replicated twice. Pulse beetle mortality rate was measured by physically counting dead weevils at 10 and 30 days after exposure to the treatment.
The mortality counts were done during the day when the weevils are highly active due to high temperatures and relative humidity. Percent adult mortality was determined by counting the number of dead insects divided by the total number of insects introduced multiplied by 100.
Grain loss and Germination test
Grain loss assessment was determined by using hundred Grain Method (HGM) as follow:

Mass of 100 grains at the beginning of the storage period will be compared with mass of 100 grains after 48 days intervals during the experiment.
Data Analysis
Percentages and mean mortality and migration rate of adult insect pest which occurred was calculated. Mean separation based on least significance difference (LSD) test and ANOVA were conducted using SAS software version 9.2.
Result and Discussions
Repellence test of pulse beetle against fennel seed and koseret leaf powders is indicated in Table 2.All the treatments have shown high repellence against pulse beetle at all amounts of treatments (5, 10, and 15grams) in the whole durations. However, there was no significant difference among treatments. Various botanical products and their extractives works as repellent has been reported by several researchers against C. chinensis L.[14-16] tested effect of plant powders and extracts against C. chinensis.
Table 2: Mean values for repellence test against pulse beetle (Callosobruchus chinensis L.) with different amounts of fennel seeds and koseret leaf powders.

Means followed by same letter within a column were not significantly different at 0.05 probability level based on LSD (Least Significance difference) test. FS: Fennel Seed Powder; KL: Koseret Leaf Powder; FS+KL: Combinations of Fennel Seeds and Koseret Leaf Powders.
Mortality of pulse beetle, C. chinensis adult at different days after treatment with 5gm, 10gm and 15gms of fennel seed and koseret leaf powders and their combined effects is presented in Table 3. Significance difference between control and treatment groups were observed throughout duration of exposure (from 10thto 20th days of treatment) indicating that both koseret leaf and fennel seed powders can be used as biological control of pulse beetle. Significantly the highest pulse beetle mortality rate (60%) was observed for koseret leaf powders followed by the admixture of koseret leaf and fennel seed powders during 20th days of treatment indicating toxicity effect of these powders on pulse beetle. It was found that the percent mortality was increasing proportionately with the increase of amount of powders and exposure time.
Table 3:Weevil mortality rate (%) after 10 and 20 days as pulse beetle (Callosobruchus chinensis L.) treated with different amounts of fennel seed and koseret leaf powders in stored chickpea.

Means followed by same letter within a column were not significantly different at 0.05 probability level based on LSD (Least Significance difference) test. Small letters: significance within column; capital letters: significance within row. FS: Fennel Seed Powder; KL: Koseret Leaf Powder; FS+KL: Combinations of Fennel seeds and Koseret Leaf Powders.
The result of this study is in accordance with the findings of
many authors Singh, Kumari and Singh, Aslam, Umrao and Verma,
Singh & Boeke [17-22] who concluded that plant dusts were proved
to be equally effective against bruchids in respect of control of
number of eggs laid, number of adults emergence, reduction in
damage to grain by the pest, weight loss, moisture content and even
in germination of the seed. DARP [23] also reported that malathion
resistance in stored product insect pests was found from all over
the world and currently, there are 122 insect-pest species, which
are found as resistant to this insecticide.
The Effect of different concentrations of fennel seed and
koseret leaf powders on chickpea seed damage as weight loss and
germination loss caused by pulse beetle was presented in Table 4.
Significance difference between control or untreated group and
treatment groups for both weight loss and germination potential of
chickpea seeds. The highest percentage weight loss was recorded in
control (33.79%) followed by 5gm fennel seed powder (16.04%).
The lowest percentage weight loss (2.92% and 3.45%) was found
in 15gm koseret leaf and fennel seed powders respectively. It was
observed that there was no significance difference in weight loss
and germination potential of chickpea seeds among 5gm, 10 gm and
15 gm treatments indicating that treatment with fennel seeds and
koseret leaf powders did not affect the quality of chickpea seeds.
This result was in agreement with findings of Chowdhury, Regmi &
Dhoj [24,25] who reported seeds treated with botanical powders
reduced the number of damaged seeds. Baral [26] reported more
number of populations and more weight loss (21.7 %) even
morethan control (16.55 %) in Azadirachta indica leaf powder
treated grains, where as in Acorus calamus, it was nil. A. calamus
was found even more effective than malathion in controlling S.
oryzae in wheat seed storage.
Table 4: Grain loss and germination test due to damage by pulse beetle (Callosobruchus chinensis L.) on chickpea (Cicer arietinum L.) seeds after 30 days of treatment with fennel seed and koseret leaf powders.

Means followed by same letter within a column were not significantly different at 0.05 probability level based on Tukey HSD (Honestly Significantly Different) test. FS: Fennel Seed Powder; KL: Koseret Leaf Powder; FS+KL: Combinations of Fennel Seeds and Koseret Leaf Powders.
Shivanna [27] studied A. calamusat 0.5, 1.5 and 2.5 g-50g of seeds as prestorage treatments against C. chinensis on red gram (Cajanus cajan). They measured fecundity; adult emergence and percent grain weight loss and found that the A. calamus powder applied at all 3 rates gave maximum protection against all 3 generations of the pest. Similarly moisture percent and germination percent were highly maintained in chickpea treated by Sesamum oil (13.10; 95.00), C. camphoraballs (13.57; 93.67) and A. calamus rhizome dust (13.15; 93.33) till the end of the experiment as initial moisture percent and germination percent of chickpea was 12.30 and 96.00 respectively. A. indicaleaf dust was poor for maintaining moisture percent (18.03) and germination percent (31.67). Malathion, too, could not show better performance in maintaining moisture percent (17.08) andgermination percent (33.67). X. armatum fruit dust was intermediate among them. Pandy & Singh confirmed that the Neem powder reduced the damage of the beetle. Al Lawati [28] proved the great inhibiting capacity of the powder extract of Annona squamosa L. on C. chinensis. Severalmanioc and bean plants tested on the Coleopters of theBruchidae type, showed an insecticidal and ovicide effect[29].
Mihret Alemayehu & Emana Getu [30] study on germination of chickpea seeds treated with botanicals tested after 90 days of the experiment reported that all the botanical treated seeds showed significantly higher germination that ranged from 80.39% to 100% compared to the untreated check (66.67%). Noug oil and primiphos-methyl treated grains gave 100% germination followed by Lemon oil (98%) and neem seed (96.08%). Thus, the result indicated that chickpea seeds treated with botanicals after 90 days of application germinated well [31].
Conclusion
The use of natural substances for pest control in agriculture is, economically, a viable option and has benefits for both the human being and the environment, due to its low persistence and toxicity. All the treatments tested were statistically superior and provided a better protection against C. chinensis compared to the untreated chickpea. However, the results revealed that powdered form of fennel seed and koseret leaf were effective against the Bruchids. Botanical materials resulted significant difference over other treatments in terms of adult mortality, adult emergence, percent grain damage, percent weight loss, moisture content and germination percent. Both fennel seed and koseret leaf powders can be used as an alternative control option in integrated storage pest management strategies. Further studies are required to check the effectiveness of repellence and pesticidal activity of fennel seed and koseret leaf powders as repellence of foliar insects; further studies to be conducted to select the most effective botanical pesticides; Studies are also required to identify biopesticides with minimum effect on seed on germination; biopesticides have great potential application in integrated pest management, due to customer preferences, health and environmental issues. Thus, studies are required to optimize biopesticide application and their control efficiency, and various form of botanicals either in the form of crude extracts, oils, and various formulations with other ecofriendly pesticides.
References
- Kannaiyan S (1999) Bioresource technology for sustainable agriculture.Associated Publishing Company. New Delhi, India,pp. 422.
- Ercan RH, Koksel A, Dag A (1995) Cooking quality and composition of chickpea grown in Turkey.20:289-293.
- Ali SI, Rabi PR (2002) pulses: chickpea, lentil, Lathyrus and French bean. In:Prasad R (Eds.),Textbook of Field Crops Production. New Delhi: Directorate of Information and Publication of Agriculture, Indian Council of Agriculture Research pp. 317-371.
- Ahmed KS, Itino T, Lchikawa T (2003) Duration of developmental stages of Callosobruchus chinensis(Bruchidae: Coleoptera) on Azuki bean and the effect of neem and sesame oils at different stages of their development. Pak J Biol Sci 6(10):332-335.
- Michalaki MP, Athanassiou CG, KavallieratosNG, Kavallieratos YA, Balotis GN (2006) Effectiveness of Metarhiziumanisopliae applied alone or in combination with diatomaceous earth against Triboliumconfusum Du Val larvae: Influence of temperature, relative humidity and type of commodity. Crop Prot25:418-425.
- HalsteadDGH (1963) External sex differences in stored products Coleoptera.Bull EntomolRes 54:119-134.
- Shaheen FA, Khaliq A, Aslam M (2006) Resistance of chickpea (Cicer arietinum L.) cultivars against pulse beetle. Pakistan J Bot 38(4): 1237-1244.
- Jackai LEN, AdallaCB (1997) Pest management practices in cowpea: A review. In: Singh BB,Raj DRM, Dashiell KE, (Eds.), Advances in Cowpea Research.International Institute of Tropical Agriculture (IITA) and Japan International Research Center for AgriculturalSciences (JIRCAS). IITA, Ibadan, Nigeria, pp. 240-258.
- Relinger LM, Zettier JL, Davis R, Simonaitis RA (1988) Evaluationof pirimiphos-methyl as a protectant for export grain.J Econ Entomol 81(2): 718–721.
- Dauguet S, Lacoste F, Ticot B, Loison JP, Evrard J, et al. (2006) La filièreoléagineuse se mobiliseautourde la problématique des résidusd’insecticides. Qualitéetsécurité sanitaire des aliments oléagineux, corps gras.[Theoilseed rallies around the issue of pesticide residues. Quality and safety of food oilseeds, fats crops].Lipids 13(6): 373–377.
- Carlos JSP (2006) Human exposure to pesticides: a risk factor forsuicide in Brazil. Vertigo-Revue Environ Sci 7: 18.
- Parugrug ML,Roxas AC (2008) Insecticidal Action of Five Plants Against Maize Weevil, Sitophilus ZeamaisMotsch. (Coleoptera: Curculionidae).KMITL Sci Tech J8(1): 23-38.
- Garcia JR (1990) Bioassay of Five Botanical Materials Against the Bean Weevil, Callosobruchus chinensis(L.) on Mungbean (Vigna radiata L.), Unpublished master’s thesis. University of the Philippines at Los Baños, College, Laguna. Anonymous.1992. Is India moving towards sustainable agriculture? South Link 2:10-11.
- Tripathi AK, Prajapati V, Agarwal KK, Khanuja SPS, Kumar S (2000) Repellency and toxicity of oil from Artimesiaannua to certain stored product beetles. J Econ Ent 93(1): 43-47.
- Tripathi MK, Sahoo P, Das BC, Mohanti S (2001) Efficacy of botanical oils, plant powders and extracts against Callosobruchus chinensis Linn. attackingblack gram CV. T9. Legume Res24:82-86.
- Valsala KK, Gokuldas M (2015) Repellent and oviposition deterrent effects of Clerodendruminfortunatum on the pulse beetle Callosobruchus chinensis L. Coleoptera: Bruchidae, Journal of Entomology and Zoology Studies3(4):250-255.
- Singh SC, PandeyNK, Kumar A (1996) Effect of neemsaw dust on mortality of thePulse beetle (Callosobruchus chinensis L.) on black gram (Vignamungo). Journal of EcotoxicologyEnvironmental Monitoring6(1): 69-71.
- Kumari K, Singh SN (1998) Evaluation of efficacy ofbotanicals an insecticide against pulse beetle(Callosobruchus chinenensisL.). Journal of AppliedBiology 8:138-140.
- Aslam M, Khan KA, Bajwa MZH (2002) Potency ofsome spices against Callosobruchus chinensis L.Journal of Biological Sciences 2(7): 449-452.
- Umrao RS, Verma RA (2002) Effectiveness of someplant products against pulse beetle on pea.Indian Journal of Entomology64: 451-453.
- Singh PK (2003) Effect of some oils against pulse beetleCallosobruchus chinensisin infesting pigeonpea.Indian Journal of Entomology65: 55–58.
- Boeke SJ, Barnaud C, Loon JJAV, Kossou DK, Huis AV, et al. (2004) Efficacy ofplantextractsagainst the cowpea beetle, Callosobruchusmaculatus. International Journal of PestManagement 50(4): 251-258.
- DARP (2003) Database of Arthropods Resistant to Pesticides, Resistant Pest Management atMichiganState University, USA.
- Choudhary BS (1990) Residual effect of eight vegetable oils in chickpea against pulse beetle(Callosobruchus chinensis Linnaeus). Indian of Plant Protec 18: 89-91.
- Regmi H, Dhoj Y (2011) Eco-Friendly Management of Pulse Beetle. The Journal ofAgriculture and Environment:12, Technical p. 81.
- Baral S (2002) Study on host preference and eco-friendly management of cowpea beetle(Callasobruchus maculatus F.,Coleoptera: Bruchidae) in Chitwan. MSc Thesis submitted to theInstitute of Agriculture and Animal Science, Chitwan, Nepal, India.
- Shivanna S, LingappaS, Patil (1994) Effectiveness of selected plant materials as protectantsagainst pulse beetle, Callosobruchus chinensis (Linn.) during storage of redgram.Karnataka J of Agric Sci 7(3):285-290.
- Al Lawati HT, Azam KM, Deadman ML (2002) Insecticidaland repellent properties of subtropical plant extracts against pulse beetle, Callosobruchus chinensis. Agric Sci 7(1): 37–45.
- Glitho LA, Ketoh KG, Nuto PY, Amevoin SK, Huignard J (2008)Approches non toxiques et non polluantes pour lecontrôle des populations d’insectesnuisiblesen Afrique duCentre et de l’Ouest. [Non-toxic and non-pollute approachesto control pest populations Central and West Africa].In: Biopesticide d’origineVégétale” [Biopesticides of Plant Origin] Regnault-Roger C, PhilogèneBJR,Vincent C, (Eds.), Lavoisier, Tech. andDoc., Paris,France, pp. 207–217.
- Mihret Alemayehu, EmanaGetu(2015) Callosobruchus chinensis (L.) (Coleoptera: Bruchidae)Management on StoredChickpea Using Botanicals in Amhara Region.
- SAS (2002) User's Guide: Statistics. Version 9.0, SAS Institute inc., Cary, NC, USA.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...





