Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4749
Research Article(ISSN: 2637-4749) 
Effect of Periparturient Intranasal Vaccination on Post Parturient Health Parameters in Holstein Cows Volume 3 - Issue 4
Cortese VS1*, Pinedo PJ2, Manriquez D2, Velasquez-Munoz A2, Solano G2, Short TH3, Cleale R3, Edwards G4, Montgomery T5, Pedraza JR3 and Bachtell R6
- 1Zoetis INC, 746 Veechdale Road, USA
- 2Department of Animal Sciences, Colorado State University, USA
- 3Zoetis INC, Parsippany, USA
- 4sup>1070 Kenilworth Ave. Napoleon, USA
- 5Maple Row Dairy, Saranac, USA
- 6Bachtell Veterinary Service, Mercersburg, USA
Received: February 18, 2020; Published: February 27, 2020
Corresponding author: Victor Cortese, Zoetis INC, 746 Veechdale Road, Simpsonville, KY 40067, USA
DOI: 10.32474/CDVS.2020.03.000167
Abstract
The objective of this study was to evaluate the effect on health and reproductive performance of vaccinating Holstein cows with an intranasal modified live viral vaccine (INV) during the peri-parturient period. Two conventional, free stall dairies and one organic certified dairy were enrolled in the study. In the two conventional dairies cows were vaccinated 18-24 days prior to expected parturition. In the third herd cows were vaccinated 18-24 days prior to expected calving date, within twelve hours after parturition or at both time points. Cattle were blocked based on parity group and expected calving date and randomized to the experimental treatments (vaccination and control) within blocks. Health and reproductive outcomes were monitored and compared to matched, randomly assigned control cows. Results from the first two dairies were used to determine power calculations for the final study in herd 3. The greatest effects of vaccination were decreases in total cows removed from the herd throughout the lactation, decreased presentation of retained fetal membranes, and lower incidences of pneumonia and mastitis. Overall, a greater impact was determined with two doses and, if one dose was administered, the results tended to favor administration at calving.
Keywords: postpartum health, postpartum immune suppression, calving stress, intranasal vaccination, immunomodulation
Abbreviations: RFM: Retained Fetal Membranes; BRSV: Bovine Respiratory Syncytial Virus; CON: Non-Vaccinated Negative Control; AI: Artificial Insemination
Introduction
The transition period is a critical time for cow health, survival,
and subsequent fertility. While some of the post-partum diseases
are more nutritional in cause (hypocalcemia, ketosis, displaced
abomasum), others have more of an immunological/infectious
underlying cause (retained fetal membranes [RFM], uterine
infections, and pneumonia). Incidences of these diseases and their
multifactorial nature have been described in detail [1,2] and a more
recent study by McNeel [3] demonstrated that there is also a genetic
component to most postpartum health disorders.
In addition to hormonal and nutritional changes, and
associated with those factors, cows going through the transition
period display pronounced systemic immune suppression [4].
Immunosuppression in periparturient dairy cows has been well
documented and specific dysfunction of both lymphocytes and
neutrophils in both periparturient cows and sows, has been
demonstrated [5-7]. This suppression of the immune system
has been associated with increased susceptibility to infectious
diseases in many systems including respiratory, reproductive and
gastrointestinal organs [8-12].
While increased immune suppression can be determined
beginning three weeks prepartum [13,14], broad immune
suppression is more dramatic in the first two weeks postpartum [15-23]. While the exact causes of immune suppression in these
animals are not clear, hormonal and metabolic changes are thought
to influence the reduction seen in systemic immune responses.
Little research has characterized the mucosal respiratory
immune response of adult cattle in the peripartum period. However,
a recent study by Cortese [24] showed that both antigen specific
and antigen nonspecific nasal immune responses of mature dairy
cows following intranasal administration of a modified live viral
vaccine [bovine respiratory syncytial virus , bovine herpesvirus
-1 and parainfluenza virus type 3; Inforce 3, Zoetis, (INV)] was
higher in cows vaccinated on the day of calving than in cows
receiving the vaccine 2-3 weeks prior to calving. Consequently,
this study examined the health and reproductive performance of
cows administered intranasal modified live viral vaccine during the
periparturient period when compared to unvaccinated controls in
three U.S. dairy herds.
Material and Methods
Study population
This study was conducted using a randomized complete block experimental design. Pregnant Holstein cows were eligible for enrollment and the blocking criteria included parity grouping and expected calving date. Each block consisted of two (studies 1 and 2) or four (study 3) treatment groups and animals were randomly assigned to cows within a block.
Treatment protocol in Dairies 1 and Dairy 2
Table 1: Distribution of cows by study location, treatment, and parity category*.

*First parity cows were heifers at time of INV administration,
parity ≥2 cows were in the dry period after completing their
prior lactation.
**Intranasal vaccine (INV) administered 14-21 days pre-calving
(PC) ***see Table 2.
Dairy 1 (D1) and dairy 2 (D2) were conventional free-stall dairies located in the Northeast (D1) and the Midwest (D2). Cow information was obtained from on-farm software and animals were blocked by parity and expected calving date and then randomly assigned into 1 of 2 treatment groups: 1) non-vaccinated negative control (CON); or 2) intranasal vaccination before calving (PC). Individual enrollments occurred when cows were moved to the close-up pens 3 weeks before expected calving date and the treatments were administered 18-24 days before expected calving date. Separated close-up pens with headlocks were used to house CON or PC cows. Pens for each treatment group were on opposite sides of a central feed delivery alley and were separated by approximately 20 feet of free air space to prevent nose to nose contact and cross-contamination of feed, and water, and was intended to prevent drift of aerosolized vaccine from vaccinates to CON cows. No direct inter-treatment contact was allowed for a minimum of 2 weeks post-treatment. Halfway through the study pens were switched for each group. Cows that calved less than seven days after treatment administration were excluded from the statistical analysis. All the health events were recorded in a commercial dairy software program (Dairy Comp 305). Animal numbers are displayed in Table 1.
Treatment protocol in Dairy 3
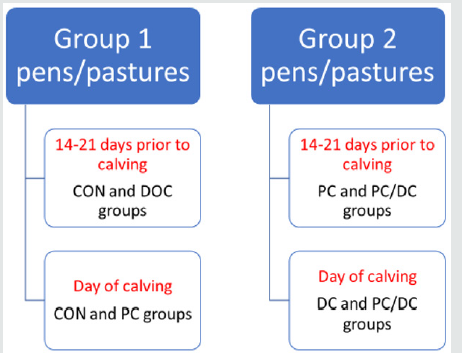
Dairy three (D3) was a large, organic certified dairy located in the Southwest (Table 1). Only cows that had completed at least one lactation were included in the study. Based on Cortese et al. (2017), three vaccination groups were considered in this location. Animals were individually identified and blocked by parity and expected calving date and randomly assigned into 1 of 4 treatment groups: 1) non-vaccinated negative control (CON); 2) intranasal vaccination at 18-24 days prior to calving (PC); 3) intranasal vaccination at the day of calving within twelve hours of parturition (DC); or 4) intranasal vaccination at both 18-24 days prior to calving and at the day of calving (PC/DC; Table 2). Once treatments were administered, the groups were separated to avoid nose-to-nose, feed, and water contact, and a minimum of 25 feet of free air space was maintained between pens/pastures for 2 weeks post-vaccination to avoid potential cross vaccination among groups. This requirement allowed for two groups to be housed together at each vaccination point: Negative control and cows vaccinated at calving were able to be housed together as could cows vaccinated pre-partum with those vaccinated prepartum and at calving until vaccination were administered at calving (Figure 1). Cow records were maintained on a commercial dairy software system (PC Dart).
Vaccine administration
Experimental vaccination programs were administered while cows were in the close-up pen and/or at the day of calving. The intranasal vaccine utilized in this study contained modified live bovine respiratory syncytial virus (BRSV), modified live, temperature sensitive bovine herpesvirus -1 (BHV-1) and modified live, temperature sensitive parainfluenza virus type 3 (PI3 V; Inforce 3, Zoetis). The vaccine was administered according to label directions with 2mL in one nostril with cannulas provided by the manufacturer. All vaccines were administered by, or under the supervision of a veterinarian. In dairies 1 and 2, vaccination was administered 18-24 days prior to calving. On D3, vaccination as administered 18-24 days prior to calving, on the day of calving (within 12 hours of giving birth) or at both timepoints.
Routine vaccination programs in the study dairies
Vaccines routinely used by the dairies were continued. Dairy 1 vaccinated cows according to the following program: At approximately 60 days prior to calving, all cows were vaccinated a viral/bacterin scours vaccine and a core E. coli mastitis bacterin. Eighteen -24 days prior to calving all cows received a core E. coli mastitis bacterin. Heifers were also administered a viral/bacteria scours vaccine.
Dairy 2 utilized the following vaccines administered to all cows: At approximately 60 days prior to calving cows received a Mannheimiahaemolyticabacterin-luekotoxoid, an autogenous Salmonella core bacterin, and a core E. colimastitis bacterin. Eighteen to twenty-four days prior to calving an autogenous Salmonellacore bacterin, a viral/bacterin scours vaccine and a core E. colimastitis bacterin were administered. At approximately 21 days in milk a modified live 5-way viral vaccine in combination with a five-way leptospira vaccine, Mannheimiahaemolyticabacterin-luekotoxoid and a core E. colimastitis bacterin were administered.At approximately 200 days of pregnancy cows were vaccinated with a modified live 5-way viral vaccine in combination with a 5-way Leptospiravaccine and an autogenous Salmonella core bacterin
Dairy 3 vaccination program included a modified live 5-way viral vaccine in combination with a 5-way leptospiravaccine and a core E. colimastitis bacterin administered 23-29 days in milk, and a 5-way leptospiravaccine at pregnancy diagnosis. At approximately 60 days prior to calving, all cows were vaccinated a viral/bacterin scours vaccine, an 8-way clostridial vaccine and a core E. colimastitis bacterin. Eighteen -24 days prior to calving all cows received a core E. colimastitis bacterin.
Breeding programs
Both D1 and D2 utilized timed artificial insemination (AI) programs. Dairy one was on a presynch, ovsynch program that included a single dose of a prostaglandin with a resynch program on open cows. Dairy 2 applied a double ovsynch program for the first service followed by an ovsynch program with pedometerbased selection on successive breeding. Cows in D3 were subject to a reproductive program based on AI following visual estrus detection. Cows’ tail heads were painted daily with color chalk and checked for estrus by removal of tail chalk. If estrus was determined, cows were inseminated during the morning. None of the cows were subjected to any type of estrus or ovulation synchronization protocol. Cows with more than 5 AI or more than 180 DIM were moved to a pen with a clean-up bull. The Voluntary waiting period was 45 d and pregnancy was diagnosed by transrectal palpation at 60 d after AI.
Statistical analysis
Following enrollment and experimental treatment administration, complete records for each cow were maintained in Dairy Comp 305 or PCDART. Data regarding lactation performance, reproductive events, disease occurrence, mortality, therapeutic treatments and cows sold or that died were collected for each enrolled cow until they either left the herd or completed the lactation. For dairies 1 and 2 mastitis cases were defined as: 1. an initial case in the cow and 2. reoccurrence in the same quarter or a different quarter >7 days from the initial case. For dairy 3 mastitis cases were defined as: 1. an initial case in the cow and 2. reoccurrence in the same quarter or a different quarter >14 days from the initial case. There was no attempt to mask farm personnel regarding experimental treatments assigned to each cow. Since all observations were observed on individual cows, animal was the experimental unit. The model for all traits included the fixed effect of experimental treatment, age, and their interaction and the random effect of month of freshening. The PROC MIXED procedure of SAS was utilized to evaluate continuous data such as 305ME and test day milk yields and log-transformed somatic cell counts. Sums of squares were partitioned to assess effects due to the random effect of block (defined as the month/year the animal freshened) and the fixed effects of treatment group, parity group, and the parity group by treatment interaction. For these analyses a covariate was included that adjusted treatment least squares means based on the potential of each animal to produce milk. Variables used in this regard were either PTA for milk (springing heifers), or 305 ME deviations for each cow within the herd (cows with a lactation record). In the case where treatment means were computed based on repeated observations (e.g., mean milk yield over 43 weeks based on weekly milk yields), the effect of week of lactation was added to the model as a fixed effect, as was a three-way interaction of treatment by lactation by week Figure 2.
Results
Cow Survival
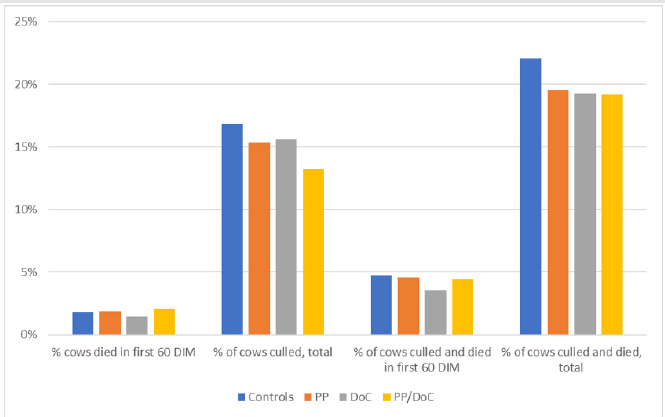
Table 3 presents the frequencies of cows that left the herd within the first sixty days of lactation and throughout the entire lactation by dairy and vaccination group. In two of the three dairies (D2 and D3), decreases, in total cow removal throughout the lactation, were seen with periparturient vaccination. While not significant, the greatest treatment effect was seen in D2 (4%, P = 1.2). In D3 vaccination during the peri-parturient period significantly decreased the total cows that were culled and died from 22.1% to 19.4% (P = 0.05) during the lactation following vaccination. A significant reduction in total cows that died during the lactation was determined with any INV schedule (P = 0.028), with the group that received INV on the day of calving having the lowest death loss. Also, the number of cows culled was lowest for PC/DC, followed by cows that received at least one INV dose and highest for the negative control group (P < 0.11). While not significant, the number of cows culled in first 60 days in milk, the total number of cows sold, and the total number of cows sold and culled improved if the INV vaccination was administered and improved as vaccination was moved to day of calving and with two doses (Figure 2). There was a 3.7% decrease in total cows culled and a 2.86% decrease in total cows removed from the herd if INV was administered twice Table 4.
Table 3:Effect of vaccination on cow survival by study location.

*Intranasal vaccine administered 14-21 days pre-calving (PC)
**All three vaccinate groups included in summary analysis of dairy 3.
Table 4: Cow removal from dairy three by vaccination group.

1CON = negative control; PC = 21-days prepartum; DC = at calving; PC/DC = 21-days prepartum and at calving; treatment effect p-value P < 0.55 for first 60 DIM and P < 0.26 for total lactation
Mastitis
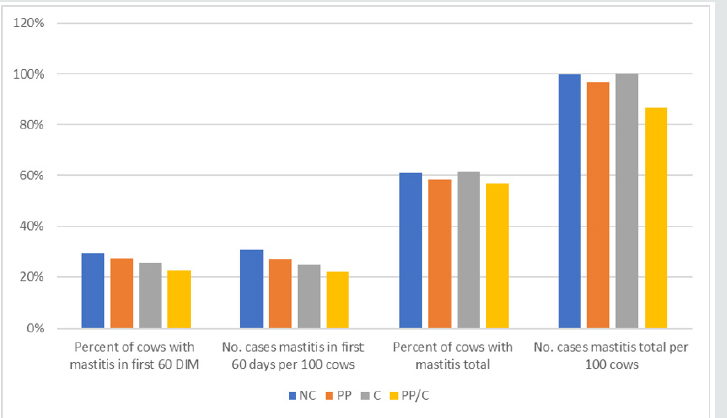
Table 5 shows mastitis incidence by treatment group. Decreases in mastitis, while not significant were seen in both dairy 1 and 2 in the first 60 days of milk and throughout lactation in INV vaccinated cows. In dairy 3, significant differences were detected between treatment groups for percent of cows (P < 0.014) and number of mastitis cases (P < 0.0043) within the first 60 days in milk and throughout the entire lactation. For each treatment group, the number of cases per 100 cows per lactation was high, indicating that a significant proportion of cows had more than one case of mastitis. The number of cases /100 cows, both in the first 60 days and throughout the lactation, were significantly higher for the CON group. Cows receiving two INV doses had the lowest incidence, followed by cows vaccinated at calving and then cows receiving the 21-day pre-partum dose (Table 6 & Figure 3). When considering the entire lactation, the same general trend occurred with the negative control group having the greatest number of cows with at least one case of mastitis, the twice dosed treatment group had the fewest number of cows and the single dose groups was in between (P < 0.10). When considering the number of cases across the entire lactation, there was a significant treatment effect with twice dosed cows having the fewest cases per 100 cows (86.5) and the negative controls (99.8) and cows vaccinated at calving (99.9) then highest (P < 0.0049) (Table 7).
Table 5:Effect of vaccination on incidence of clinical mastitis.

*Intranasal vaccine (INV) administered 14-21 days pre-calving (PC)
**All three vaccinate groups included in summary analysis of dairy 3.
Table 6: Mastitis incidence in dairy 3 by treatment group.

1CON = negative control; PC = 21-days prepartum; DC = at calving; PC/C = 21-days prepartum and at calving 2For percent cows with mastitis first 60 days, PC/C differed from CON (P < 0.0018) and PC (P < 0.0265) and DC differed from CON (P < 0.0688); for number cases mastitis first 60 days, PC/C differed from CON (P < 0.0004) and PC (P < 0.0296) and DC differed from CON (P < 0.0277); for percent cows with mastitis total, PC/C differed from CON (P < 0.060) and DC (P < 0.0301); and for number of total cases mastitis, PC/C differed from CON (P < 0.0017), DC (P < 0.0015) and PC (P < 0.0167).
Table 7: Mastitis incidence in dairy 3 by treatment group.

*Intranasal vaccine administered 14-21 days pre-calving (PC).
1Number of retained fetal membranes too low for statistical analysis).
**All three vaccinate groups included in summary analysis of dairy 3.
Postpartum Reproductive Health
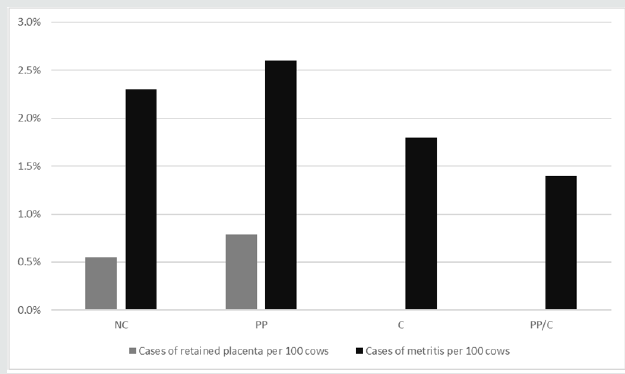
The frequency of reproductive disorders was lower for vaccinated cows in D1. While the number of RFM decreased with INV vaccination in D3, the extremely low number of RFM in this herd made statistical evaluation impossible. Dairy 3 metritis levels were high enough for evaluation; however, while INV vaccination decreased the percent of uterine infection by over 2%, there was no statistical difference. Figure 4 shows the impact on incidence of metritis and RFM by vaccination group for D3 (Table 8).
Table 8: Impact of vaccination on reproductive health disorders by vaccination group- dairy 3.

1CON = negative control; PC = 21-days prepartum; DC = at calving; PC/C = 21-days prepartum and at calving *Number of retained fetal membranes too low for statistical analysis.
Post-Partum Pneumonia and other Health Disorders
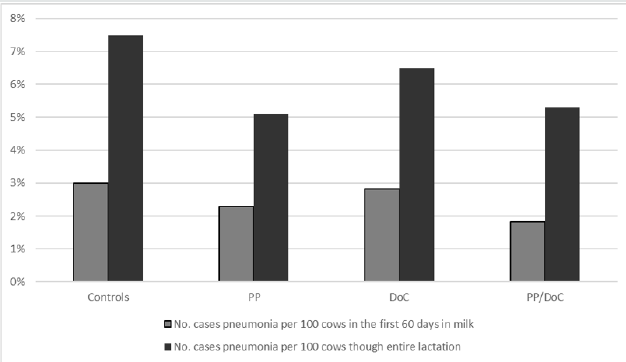
Cows vaccinated in D3 exhibited decreases in pneumonia that were only significant when evaluated throughout the entire lactation (Table 9). While not significant, some reduction in the incidence of pneumonia was determined in D1. It is interesting to note, that while not significant, D2 had a higher level of pneumonia in the vaccinates. Figure 5 shows the incidence of pneumonia by vaccination group for dairy 3. Treatment comparisons for other health disorders for D3 are shown in Table 10. Most of the health events were at relatively low frequencies with no differences between treatment groups in all three dairies. One exception to this was the incidence rate of lameness in D3. While not significant (P = 0.09), vaccinated cows trended toward lower levels of lameness.
Table 9: Impact of vaccination on incidence of pneumonia by dairy.

*Intranasal vaccine administered 14-21 days pre-calving (PC).
**All three vaccinate groups included in summary analysis of dairy 3.
Table 10:Dairy 3 summary of other health events incidence rates by vaccination group.

1CON = negative control; PC = 21-days prepartum; DC = at calving; PC/C = 21-days prepartum and at calving.
Reproductive Performance
Reproductive outcomes for the timed artificial insemination herds and for the herd using estrus detection were analyzed. No differences were seen between the treatment groups.
Milk yield and somatic cell count outcomes for D3 are shown
in Table 11. Somatic cell count information was not available for dairy 2. It is interesting to note that, despite significant decreases in mastitis, none of the dairies showed an increase in milk production in the vaccinates. In dairy 3, only two variables were significantly different in the yield and somatic cell count variables analyzed. These were test day milk yield in the first and third test days with the group receiving INV at calving having the lowest yields and negative control group the highest yield and the prepartum and twice vaccinated groups in between. Even though differences were detected on these two test days, the difference was not present for either the 2nd, 4th test days and total production.
Table 11:Summary of milk yield and somatic cell count variables – Dairy 3.

1CON = negative control; PC = 21-days prepartum; DC = at calving; PC/C = 21-days prepartum and at calving
2For test day 1, DC differed from CON (P < 0.0005), PC (P < 0.0171) and PC/C (P < 0.1042); CON differed from PC/C (P < 0.0776).
3For test day 3, DC differed from CON (P < 0.0042) and PC/C (P < 0.0417) and CON differed from PC (P < 0.0185).
Discussion and Conclusion
Postpartum health problems and high replacement rates
continue to be major issues in the dairy industry. Postpartum
health has been shown to impact many aspects of profitability on
the dairy including reproduction, and longevity in the herd [25]
The causes of post calving health issues are multifactorial involving
nutrition, environment and hormonal changes. These changes can
lead to metabolic diseases and immunologic dysfunction that can
lead to secondary infections. A recent article showed that there is
also a genetic component to susceptibility to various postpartum
problems.
Immune modulators have been tested for years to decrease post
calving diseases and antibiotic usage by improving the depressed
immune system of the postpartum dairy cow. These products
have all been administered systemically and have limited success
or increased some disease parameters. Most have not passed the
research phase to be developed into products to be marketed or
have increased some postpartum disorders [26]. However, a recent
study suggested that, while the dairy cow is systemically immune
suppressed post calving, the local immune system may be spared
immune suppression or even up regulated in the postpartum cow.
Homing of immune responses bv the local immune system has been
well established [27]. Furthermore, studies have demonstrated that
the trafficking on the respiratory and reproductive immune system
are linked and share common trafficking and upregulation [28].
Intranasal vaccination has also been shown to simulate reproductive
tract protection [29] in women. The vaccine, and its components,
used in this study have been shown to not only stimulate antigen
specific protection but also release of non-specific lymphokines
and cytokines that can act as immune modulators [30,31]. Notably,
the highest immune response seen when administered on the day
of calving [32,33].
The first two studies were intended to examine the impact of
the IN vaccine on postpartum pneumonia. The timing of vaccine
administration at 18-24 was determined based on the studies
indicating that as parturition approaches, immune suppression
increases. With the publication of the afore mentioned study
indicating potential immune shifting on the day of calving, this group
was included in dairy 3. The impact on health outcomes other than
pneumonia was unexpected and helped to determine statistical
power for the third dairy. Where differences were observed, though
not statistically significant across all three dairies, percentage
improvement was close in all three dairies indicating a potential
lack of statistical power in the first two dairies. Periparturient
vaccination with the INV had a significant impact on lowering RFM
and metritis (dairy 1), total cattle removed during the lactation,
mastitis in the first 60 DIM and throughout lactation, pneumonia,
and lameness. An unexplained increase in ketosis was seen in
dairy 2 in the vaccinate group. Outcomes improved as vaccination
was moved from 18-24 days prior to calving to the day of calving
and generally the best results were seen with the vaccine was
administered twice. It is interesting to note that this response was
not significant (~.5% decrease in the first 60 days) but the impact
on total removals continued to increase during the entire lactation
(Table 2).
Collectively, this set of studies indicates that using this INV
during the periparturient period can improved several postpartum
health outcomes due to the up regulation of the immune system
and supports earlier work indicating better immune responses
when administered on the day of calving. While this vaccine can be
used for immune modulation, more importantly this study suggests
that future immune modulators may have a better outcome if
administered to the local immune system.
References
- Ingvartsen KL, Dewhurst RJ, Friggens NC (2003) On the relationship between lactational performance and health: is it yield or metabolic imbalance that cause production diseases in dairy cattle? A position paper. Livestock Production Science 83(2-3):277-308.
- LeBlanc SJ, Lissemore KD, Kelton DF, Duffield TF, Leslie KE (2006) Major advances in disease prevention in dairy cattle. J Dairy Sci 89(4): 1267-1279.
- McNeel AK, Reiter BC, Weigel D, Osterstock J, Di Croce FJ(2017) Validation of genomic predictions for wellness traits in US Holstein cows. J Dairy Sci 100(11):9115-9124.
- Burton JL, Madsen SA, Chang LC, Weber PS, Buckham KR, et al. (2005) Gene expression signatures in neutrophils exposed to glucocorticoids: a new paradigm to help explain “neutrophil dysfunction” in parturient dairy cows. Vet Immunol Immunopathol 105:197- 219.
- Kehrli ME, Nonnecke BJ, Roth JA (1989) Alterations in bovine neutrophilfunction during the periparturient period. Am J Vet Res 50:207-220.
- Kimura K, JGoff JP, KehrliME, Harp JA (1999) Phenotype analysis of peripheralblood mononuclear cells in periparturient dairy cows. J Dairy Sci 82:315-319.
- Löfstedt J, Roth JA, Ross RF, Wagner WC (1983) Depression of polymorphonuclear leukocyte function associated with experimentally induced Escherichia coli mastitis in sows. Am J Vet Res 44(7): 1224-1228.
- Mordak R, Anthony SP (2015) Periparturient stress and immune suppression as a potential cause of retained placenta in highly productive dairy cows: examples of prevention. Acta Vet Scand 57: 84.
- Cai TQ, Weston PG, Lund LA, Brodie B, McKenna DJ, et al. (1994) Association between neutrophil functions and periparturient disorders in cows. Am J Vet Res 55(7): 934-943.
- Pelan-Mattocks LS, Kehrli ME, Casey TA, Goff JP (2000) Fecal shedding of coliform bacteria during the periparturient period in dairy cows. Am J Vet Res 61(12): 1636-1638.
- Kimura K, Goff JP, Kehrli ME, Reinhardt TA (2002) Decreased neutrophil function as a cause of retained placenta in dairy cattle. J Dairy Sci 85(3): 544-550.
- Burvenich C, Bannerman DD, Lippolis JD, Peelman L, Nonnecke BJ, et al. (2007) Cumulative physiological events influence the inflammatory response of the bovine udder to Escherichia coli infections during the transition period. J Dairy Sci 90 (1): 39-54.
- Comline RS, Hall LW, Lavelle RB, Nathanielsz PW, Silverg M (1974) Parturition in the cow: endocrine changes in animals with chronically implanted catheters in the foetal and maternal circulations. J Endocrinol 63: 451-472.
- Harp JA, Kehrli ME, Hurley DJ, Wilson RA, Boone RA (1991) Numbers and percent of T lymphocytes in bovine peripheral blood during the periparturient period. Vet Immunol Immunopathol 28(1): 29-35.
- Guidry AJ, Paape MJ, Pearson RE (1976) Effects of parturition and lactation on blood and milk cell concentrations, corticosteroids and neutrophil phagocytosis in the cow. Am J Vet Res 37(10): 1195-1200.
- Ishikawa H (1987) Observation of lymphocyte function in perinatal cows and neonatal calves. Jpn J Vet Sci 49: 469-475.
- Nagahata H, Makino S, Takeda S, Takahashi H, Noda H (1988) Assessment of neutrophil function in the dairy cow during the perinatal period. J Vet Med B 35: 747-751.
- Nagahata H, Ogawa A, Sanada Y, Noda H, Yamamoto S (1992) Peripartum changes in antibody producing capability of lymphocytes from dairy cows. Vet Q 14: 39-40.
- Saad AM, Concha C, Åström G (1989) Alterations in neutrophil phagocytosis and lymphocyte blastogenesis in dairy cows around parturition. J Vet Med B 36: 337-345.
- Stabel JR, Kehrli ME, Thurston JR, Goff JP, Boone TC (1991) Granulocyte colony- stimulating factor effects on lymphocytes and immunoglobulin concentrations in periparturient cows. J Dairy Sci 74(11): 3755-3762.
- Dosogne H, Burvenich C, Freeman AE, Kehrli ME, Detilleux JC, et al. (1999) Pregnancy-associated glycoprotein and decreased polymorphonuclear leukocyte function in early post-partum dairy cows. Vet Immunol Immunopathol 67(1): 47-54.
- Detilleux JC, Kehrli ME, Stabel JR, Freeman AE, Kelley DH (1995) Study of immunological dysfunction in periparturient Holstein cattle selected for high and average milk production. Vet Immunol Immunopathol 44(3): 251-267.
- Heiser A, McCarthy A, Wedlock N, Meier S, Kay J, et al. (2015) Grazing dairy cows had decreased interferon-γ, tumor necrosis factor, and interleukin-17, and increased expression of interleukin-10 during the first week after calving. J Dairy Sci 98(2): 937-946.
- Cortese VS, Woolums A, Hurley DJ, Berghaus R, Bernard JK, et al. (2017) Comparison of interferon and bovine herpesvirus-1-specific IgA levels in nasal secretions of dairy cattle administered an intranasal modified live viral vaccine prior to calving or on the day of calving. Vet Immunol Immunopath 187: 35-41.
- Hossein-Zadeh NG (2013) Effects of main reproductive and health problemson the performance of dairy cows: a review. Span J Agri Res11(3):718-735.
- Ruiz R, Tedeschi LO, Sepúlveda A (2017) Investigation of the effect of pegbovigrastim on some periparturient immune disorders and performance in Mexican dairy herds. J Dairy Sci 100(4): 3305-3317.
- Mora JR, Von Andrian UH (2008) Differentiation and homing of IgA-secreting cells.Mucosal Immunol1:96-109.
- Csencsits KL, Walters N, Pascual DW (2001) Cutting edge: dichotomy of homing receptor dependence by mucosal effector B cells: alpha(E) versus L-selectin. J Immunol 167(5): 2441-2445.
- Baker JA, Lewis EL, Byland LM, Bonakdar M, Randis TM, et al. (2017) Mucosal vaccination promotes clearance of Streptococcus agalactiae vaginal colonization. Vaccine 35(9): 1273-1280.
- Gerber JD, Marron AE, Kucera CJ (1978) Local and systemic cellular and antibody responses of cattle to infectious bovine rhinotracheitis virus vaccines administered intranasally or intramuscularly. Am J Vet Res 39(5): 745-760.
- DuffGC, Malcolm-Callis KJ, Walker DA, Wiseman MW, Galyean ML, et al. (2000) Effects of intranasal versus intramuscular modified live vaccines and vaccine timing on health and performance by newly received beef cattle. BovPract34(1):66-71.
- Cook NB, Nordlund KV (2004) Behavioral needs of the transition cow and considerations for special needs facility design. Veterinary Clinics: food animal practice 20(3): 495-520.
- Ishikawa H, Shirahata T, Hasegawa K (1994) Interferon-γ production of mitogen stimulated peripheral lymphocytes in perinatal cows. J Vet Med Sci 56(4): 735-738.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...