Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4749
Research Article(ISSN: 2637-4749) 
Antibacterial Effects of Carotenoid Pigment Extracted from Rhodotorula Glutinis Strains on Staphylococcus Aureus Isolated from Mastitis Samples of Dairy Cows Volume 5 - Issue 1
Soheila Naisi1, Mansour Bayat1*, Taghi Zahraei Slehi2, Bahareh Rahimian Zarif3 and Ramak Yahyarait2
- 1Department of Pathobiology, Faculty of Veterinary Medicine, Islamic Azad University, Science and Research Branch, Iran
- 2Department of Microbiology and Immunology, Faculty of Veterinary Medicine, University of Tehran, Iran
- 3Department of Microbiology, Faculty of Basic Sciences, Islamic Azad University, Sanandaj Branch, Iran
Received: September 26, 2022; Published: October 06, 2022
Corresponding author: Department of Pathobiology, Faculty of Veterinary Medicine, Islamic Azad University, Science and Research Branch, Tehran, Iran
DOI: 10.32474/CDVS.2022.05.000205
Abstract
Background: Staphylococcus aureus is the most common cause of bovine mastitis, which is often difficult to cure and leads to a lot of economic losses. Therefore, new antibacterial compounds (Especially natural compounds) are being investigated as an alternative to treat infections caused by these bacteria.
Objective: The aim of this study was to investigate the effect of pigments extracted from Rhodotorula against planktonic growth of S. aureus isolates.
Methods & Materials: In this descriptive research study, 100 milk samples were taken from clinical mastitis cow and isolation of S. aureus and Rhodotorula yeast was performed using standard microbiological tests. Extraction of carotenoid pigment of Rhodotorula yeast used to identify antimicrobial effect against S. aureus isolates and was measured by micro-dilution test. Scanning electron microscope (SEM) was also used to confirm the effect of the pigment on S. aureus isolates.
Results: A total of 25% of milk samples were infected with S. aureus, which confirmed by molecular method. A species of Rhodotorula was also isolated in one case which was confirmed as R. glutinis by PCR method (identification of its gene). The Geometric mean of MIC (Minimum Inhibitory Concentration) and MBC (Minimum Bactericidal Concentration) values of the carotenoid pigment was 39.36 and 78.82 μl.ml-1, respectively.
Conclusions: In general, we showed that carotenoid pigment from R. glutinis can be effective agents planktonic form of S. aureus isolates. Therefore, it can be claimed that the carotenoid pigment Rhodotorula is a good candidate for controlling Staphylococcus infections. However, many studies and clinical trials should have been performed on it.
Keywords:Mastitis; Staphylococcus aureus; Rhodotorula glutinis; Pigment; SEM
Introduction
Mastitis is one of the most common and economically important diseases in dairy cows in the world, so it causes a lot of damage to the livestock industry every year. It considerably affects milk production, animal welfare, and food safety [1]. More than 140 different pathogenic species have been reported in scientific papers as factors in mastitis [2]. The majority of mastitis cases are produced by a relatively small group of bacteria, including Staphylococcus aureus, Streptococcus aureus, Mycoplasma spp and coliforms [1,3].
S. aureus is one of the most important and common bacteria in the clinical development and subclinical mastitis [4,5]. It is also considered as one of the incriminated pathogenic in humans and causes numerous skin and systemic diseases. Beta-lactams are the most successful group of antibiotics ever used to treat mastitis infections caused by S. aureus isolate. Unfortunately, resistance toward all B-lactam antibiotics has eventually been observed [6-8], therefore, new complementary treatment strategies (Nanoparticles, photodynamic therapy, herbal compounds, microbial pigments and ...) seem to be effective against resistant pathogens [9]. Among antimicrobial compounds, bacterial pigments are great alternatives to antibiotics because resistance toward them has not been reported among microorganisms, therefore it has attracted the attention of many researchers [10]. The pigments produced by microorganisms includes monascins, violacein, indigo, melanin, flavins, quinones, and more specifically carotenoids, showed distinct antibacterial effect against many pathogenic bacteria [11]. Numerous microorganisms have the ability to produce pigments. However, actinobacteria, rhodococcus and a number of fungi and yeasts including Rhodotorula, have the highest ability to produce pigments [12,13].
Rhodotorula glutinis is one of the most important and useful fungi in the production of various pigments [12]. This yeast has been used industrially in the production of carotenoid pigments and as a biological control agent for post-harvest diseases of fruits. Carotenoids belong to the chemical group of isoprenoid and fat-soluble polymers [14]. This pigment acts as a light-absorbing chromophore and produces yellow, red and orange colors [14,15]. Therapeutic effects of carotenoids include their use in the treatment of cancer, the treatment of cataracts and the treatment of cardiovascular diseases. However, few studies on the antibacterial properties of this pigment have been done sparsely in the world [15]. Therefore, in this study, we tried to measure the antimicrobial effect of Rhodoturola pigment on S. aureus isolates, so that if there is a sufficient antimicrobial effect, more research can be done in this regard.
Materials and Method
Sample collection and yeast isolation
To isolate Rhodotorula Spp, different sediment samples (including soil, sludge, plants and flowers) were collected from around the Tehran University yards. From each sample 100 g were placed in plastic bag with an ice bag and transferred to microbiology laboratory in Tehran Veterinary college and then processed immediately to isolate yeast. Ten g of each sample was homogenized in 90 ml of 9% sterile saline, then cultured on yeast-peptone-dextrose (YPD) agar extract. All plates were incubated at 28 °C for 24 hours to grow the yeast colony and determine its morphology [12].
Molecular identification of yeast isolates
Molecular method was used to confirm the species suspected of Rhodotorula. To do this, First, DNA of isolates was extracted using phenol-chloroform method, then a fragment sequence from ribosomal genome (ITS) was amplified and identified using a specific primer. Primer sequence and PCR product length are given in Table 1. PCR reaction was performed in a final volume of 25 μl including 12.5 μl of Mastermix (Amplicon, 2X), 0.5 μl of each primer (10 picomoles), 3 μl of template DNA and 8 μl of deionized water. The PCR amplification was performed under the following conditions: initial denaturation at 95°C for 8 min followed by denaturation at 95°C for 1 min, annealing at 58°C for 45 sec and extension at 72°C for 1 min (35 cycles) and a final extension at 72°C for 10 min. Finally, the PCR product was run on 1% agarose gel for 45 minutes [16]. The sti gene of Rhodotorula species was also sequenced using the Sanger method.
Sample Collection and S. aureus isolation
In total, 100 samples of cow’s milk suspected of mastitis were taken from industrial farms in Famenin city in Hamedan province of Iran, over a period of three months and transferred to a microbiology laboratory. Milk samples were centrifuged at 4 °C at 8000 rpm for 10 minutes and the sediment was cultured using swap in blood agar medium. After 24 hours of incubation, S. aureus colonies were identified using standard biochemical tests such as catalase, nitrate, and DNase and coagulase test [17,18].
Molecular identification of S. aureus isolates
For molecular confirming of S. aureus isolates, S. aureus species- specific primer was used. First the DNA of bacteria was extracted by boiling method, then primers designed by Mehrota were used to amplify the femA gene (Tables 1) [19]. PCR reactions and temperature program were performed as in section 1-1, only the annealing temperature was 58 °C.
Purification of the carotenoid pigment
First, mass cultivation of Rhodotorula yeast was carried out in MMS medium and placed in a shaker incubator at 25 º C, 200 rpm for 86 hours. The yeast cells were then centrifuged at 5000 rpm for 20 minutes and then were washed. To the obtained biomass was added a Hydrochloric Acid, 1.00 Normal, and transferred to a bain-marie at 70℃ for 90 minutes. After acid removal by washing, the cells were immersed in a solution of acetone and methanol (1: 1) for 24 hours. Eventually, the crude pigment was collected and concentrated by evaporation. Quantity of crude pigment was measured by adding the pigment into the dried 25 ml preweighed beaker. After evaporation of the solvent, the weight of crude pigment was measured and stored in sterile vial.
Evaluation of antimicrobial effect of Rhodotorula pigment
The Minimum Inhibition Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of ethanolic extract of pigment were evaluated by well micro-titer plate method according to the guidelines of CLSI 2018. Briefly, 100 μL of the bacterial inoculate (corresponding to 0.5 of the McFarland) and 100 μL of the two-fold serial dilutions of the pigment (0.25 to 256 μL. mL-1) were distributed into each well of ELISA microplates (96 well). Dilution was done till tenth. Column eleventh, as a negative control, contained dimethyl sulfoxide (DMSO) and bacteria, and column twelfth, as positive control, contained DMSO, bacteria and Penicillin (1 mg/ mL). After 24 hours of incubation of microplates at 37 °C, microtiter plates were visually scored. MIC is the minimum concentration at which microbial growth is inhibited. For determining the MBC, 0.1 ml of culture medium from each well was sub-cultured on Muller Hinton agar plate. The MBC was considered as the lowest concentration of the pigment associated with no bacterial culture.
Electron microscope
Electron microscopy was used to evaluate the effect of carotenoid pigment of Rhodotorula glutinis on S. aureus isolates. For this purpose, bacteria were cultured in TSB medium and after adding carotenoid pigment to the medium (final concentration of carotenoid pigment equal to MIC / 2), a sterile lamellar with a size of 0.5 cm2 was placed in the medium. After incubation of the samples for 24 hours at 37 °C, the lamellae were fixed with 2.5% Glutaraldehyde solution. The samples were then dehydrated with ethanol and dried at room temperature. The fixed bacterial samples were mixed with the desired nanoparticles and a 9 nm gold coating was applied to the samples by spattering device. Eventually, the samples were photographed by FESEM model TESCAN mira3 (made in the Czech Republic) in different magnifications with a voltage of 15 kV [20].
Results
Yeast isolation
In order to identify and isolate the Rhodotorula spp.. colony, the samples were cultured in Potato dextrose agar and incubated for 48h at 37 °C (Figure 1). Then the colonies were examined macroscopically for size, shape, color, and margin. In total, one species (1/100) of Rhodotorula was isolated from mastitis samples which was identified as R. glutinis using physical characteristics and biochemical tests. Also, in molecular confirmation, its gene was amplified, and the desired band (550 bp) was observed in electrophoresis of PCR product (Figure 2).
Isolation of S. aureus
A total of 25% (25/100) of milk samples contained S. aureus strain in biochemical tests. Also, the Molecular assay were also completely compatible with biochemical tests and in search of femA gene using PCR test, all isolates carried this gene and in PCR electrophoresis, showed a band of 132 bp (Figure 2).
Figure 2: PCR products obtained from the amplification of femA and its genes. Lane L: a 100 bp DNA ladder; lanes 1: Positive controls (S. aureus ATCC 25923, R. glutinis), lanes 2: Isolates, lanes 3: Negative controls.

MIC and MBC
Broth microdilution test was used to determine the minimum inhibitor concentrate of pigment on S. aureus isolates and the results are shown in Figure 3. The MIC and MBC of pigment on S. aureus isolates from mastitis were between 2 - 128 μg / ml. The Geometric mean of MIC and MBC) values of the pigment on S. aureus isolates was 39.36 and 78.72 μl.ml-1. The results of microdilution test showed that all isolates were susceptible to Rhodotorula pigment.
SEM
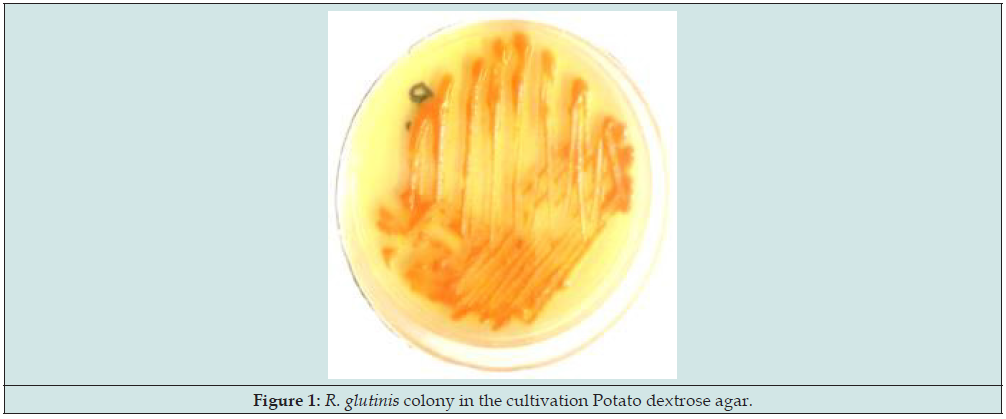
SEM was employed to determine the inhibitory effects of the carotenoid pigment on S. aureus isolates. The results of SEM analysis showed that the carotenoid pigment at the sub-MIC concentration had a destructive effect on S. aureus isolates. As shown in Figure 4 - Part A, S. aureus is placed on a glass slide in the form of a grape cluster. In this case, S. aureus is undamaged and has a healthy spherical shape. Figure 4-Part B & C, shows that the pigment destroys the bacterial cell wall by creating pores. In Figure 4-Part D, the bacterium S. aureus is completely destroyed.
Figure 4: SEM results. Part A: Grape-like clusters of S. aureus, Part B, C and D: Destruction of the cell wall of S. aureus due to the application of carotenoid pigment of R. glutinis.
Discussion
Bovine mastitis is an inflammatory response of the udder tissue in the mammary gland caused due to physical trauma or microorganism infections [21,22]. It is one of the most important diseases of dairy cows, which leads to economic loss in dairy industries due to the reduction in the quantity and quality of milk and the reduction of the production life of infected cows. S. aureus has been the most predominant bacteria causing both agents of clinical and subclinical mastitis [22,23]. In the current study, a total of 100 milk samples were analyzed by biochemical and molecular test and results indicated that 25% were found positive for S. aureus isolation. Lower frequency of mastitis-associated S. aureus has been reported from maku, Iran (8%) [24], Northeast of Iran (10.3%) [25], Japan (7.7%) [26] and Argentina (20%) [27] , in contrast with the higher frequency informed from Daland, Iran (20%) [28], Shiraz, Iran (31%) [29], Kurdistan, Iran (33.5%) [30], Bangladesh (72%) [31], Netherlands (42) [4], and India (53.3%) [7]. In general, the results of studies conducted in Iran and the world indicate the importance of S. aureus in causing mastitis in farms.
On the other hand, due to the increasing of drug resistance among S. aureus isolates, physicians and veterinarians have encountered difficulties in treating infections caused by this pathogen. Therefore, many researchers have tried to find new methods and antimicrobial compounds to control and prevent this problem [9,32]. One of the new, vital and bioactive compounds for controlling bacterial infections is the use of pigments produced by microorganisms [33]. Pigments such as carotenoids, melanins, violacein, indigo, monascins, flavins and quinones have been reported as good antimicrobial agents [34]. Rhodotorula yeast is a pigment-producing fungus. The genus Rhodotorula is abundant in nature and can be isolated from various sources such as air, seawater, plants, dairy products and the environment [35]. In a recent study, a species of Rhodotorula was isolated from milk samples taken from cows with suspected subclinical infections, which was confirmed as a species of R. glutinis in molecular studies (Identification of its gene). The pigment obtained from R. glutinis yeast was purified and prepared, and its antimicrobial effect was measured on S. aureus isolates using broth microdilution method. According to the results of the microdilution broth test, the pigment extracted from Rhodotorula had an inhibitory effect on the growth of all isolates of S. aureus and at a concentration of 80 μ/ml, was able to kill Staphylococcus isolates.
Research have shown that the antimicrobial activity of carotenoids on bacteria have not been the same. The carotenoids produced by Halomonas have been reported to have antimicrobial activity against the antibiotic resistant Klebsiella sp., Streptococcus pyogenes, Pseudomonas aeruginosa, Escherichia coli and S. aureus [36]. In contrast, carotenoids extracted from M. luteus and M. roseus did not (didn’t) have a lethal effect on Escherichia coli but inhibited the growth of Enterococcus faecalis and S. aureus. Other studies have shown that the carotenoid pigment extracted from Corynebacterium sp., Bacillus sp., Kocuria roseus, and Brevibacterium sp. had good antimicrobial effects against S. zillusus, S. aureus ATCC 25923 (MSSA) and Bacillus masserans [37].
Since gram- negative bacteria had shown high resistance to antimicrobial agents against gram- positive bacteria, it is expected that the MIC of Rhodotorula pigment against gram-negative bacteria is more than 80 μ/ml. In this case, Yolmeh et al (2016) reported that gram-negative bacteria were highly resistant to R. glutinis pigment than gram- positive bacteria [38]. In another study, Yolmeh et al (2018) Showed that the carcinoid pigment Micrococcus rosacea had a stronger destructive effect against gram-positives than gram-negatives bacteria [39]. In general, it could be said that the type of bacteria and the presence of resistance genes, including efflux pumps, which are generally resistant to many antimicrobial agents, can affect the MIC of pigments, however no specific resistance to pigments has been reported so far [40]. Using SEM is the most commonly method to study the effects of antimicrobial compounds on microbial structure [41]. In this study, SEM was also used to confirm the effect of carotenoid pigments produced by R. glutinis on S. aureus isolates. As presented in the figure 4, untreated bacterial cells had a normal shape and were arranged in clusters form, but these structures were rarely seen in pigment -treated S. aureus cells. In the treatment group (containing carotenoid pigment), S. aureus cells were deformed and a rupture in the cell wall and leakage of cytoplasmic contents were observed. The mechanism impact of carotenoid pigments produced by R. glutinis on bacteria has not been completely clear. Although researchers have shown that, natural compounds such as plant essential oils and pigments of microorganisms, nonspecifically destroy the bacterial cell wall and kill it.
Conclusion
In the present study, the prevalence of S. aureus in mammary gland quarters of dairy cows in traditional farms of Famenin city was very high, hence, it can be said that the outbreak of S. aureus leads to the economic losses for traditional farms. Therefore, it is recommended to prevent the spread of S. aureus among other cows as soon as possible. The results of antimicrobial activity of carotenoid pigments produced by Rhodotorula also showed that this substance at a concentration of 80 micrograms has a good antimicrobial effect against S. aureus isolates and can be considered a great candidate for a novel complementary treatment of MRSA infections in the future.
References
- Molineri AI, Camussone C, Zbrun MV, Archilla GS, Cristiani M, et al. (2021) Antimicrobial resistance of Staphylococcus aureus isolated from bovine mastitis: Systematic review and meta-analysis. Prev Vet Med 188: 105261.
- Pascu C, Herman V, Iancu I, Costinar L (2022) Etiology of Mastitis and Antimicrobial Resistance in Dairy Cattle Farms in the Western Part of Romania. Antibiotics 11(1): 57.
- Heikkilä A-M, Liski E, Pyörälä S, Taponen S (2018) Pathogen-specific production losses in bovine mastitis. J Dairy Sci 101(10): 9493-9504.
- Hoekstra J, Zomer AL, Rutten VP, Benedictus L, Stegeman A, et al. (2020) Genomic analysis of European bovine Staphylococcus aureus from clinical versus subclinical mastitis. Scientific reports 10(1): 1-11.
- Pérez VKC, Da Costa GM, Guimarães AS, Heinemann MB, Lage AP, et al. (2020) Relationship between virulence factors and antimicrobial resistance in Staphylococcus aureus from bovine mastitis. J Glob Antimicrob Resist 22: 792-802.
- Algammal AM, Enany ME, El-Tarabili RM, Ghobashy MO, Helmy YA (2020) Prevalence, antimicrobial resistance profiles, virulence and enterotoxins-determinant genes of MRSA isolated from subclinical bovine mastitis in Egypt. Pathogens 9(5): 362.
- Shah MS, Qureshi S, Kashoo Z, Farooq S, Wani SA, et al. (2019) Methicillin resistance genes and in vitro biofilm formation among Staphylococcus aureus isolates from bovine mastitis in India. Comparative immunology, microbiology and infectious diseases 64: 117-24.
- Hakimi Alni R, Mohammadzadeh A, Mahmoodi P, Pajohi Alamoti MR (2018) Determination of ecovars, antibiotic susceptibility and tst gene frequency in Staphylococcus aureus isolated from dairy food products. Iranian Veterinary Journal 14(3): 41-49.
- Alni RH, Tavasoli F, Barati A, Badarbani SS, Salimi Z, et al. (2020) Synergistic activity of melittin with mupirocin: A study against methicillin-resistant S. aureus (MRSA) and methicillin-susceptible S. aureus (MSSA) isolates. Saudi J Biol Sci 27(10): 2580-2585.
- Azman AS, Mawang CI, Abubakar S (2018) Bacterial pigments: the bioactivities and as an alternative for therapeutic applications. Natural Product Communications 13(12): 1747-1754.
- Venil CK, Velmurugan P, Dufossé L, Renuka Devi P, Veera Ravi A (2020) Fungal pigments: Potential coloring compounds for wide ranging applications in textile dyeing. J Fungi 6(2): 68.
- Zhao Y, Guo L, Xia Y, Zhuang X, Chu W (2019) Isolation, identification of carotenoid producing Rhodotorula sp. from marine environment and optimization for carotenoid production. Mar Drugs 17(3): 161.
- Aruldass CA, Dufossé L, Ahmad WA (2018) Current perspective of yellowish-orange pigments from microorganisms-a review. Journal of Cleaner Production 180: 168-182.
- Tuon FF, Costa SF (2008) Rhodotorula infection. A systematic review of 128 cases from literature. Revista iberoamericana de micologia 25(3): 135-140.
- Ramesh C, Vinithkumar NV, Kirubagaran R, Venil CK, Dufossé L (2019) Multifaceted applications of microbial pigments: current knowledge, challenges and future directions for public health implications. Microorganisms 7(7): 186.
- White TJ, Bruns T, Lee S, Taylor J, Innis MA, et al. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications 18(1): 315-322.
- Hakimi Alni R, Mohammadzadeh A, Mahmoodi P, Alikhani MY (2017) RAPD-PCR analysis of Staphylococcus aureus strains isolated from different sources. Comparative Clinical Pathology 26(4): 823-830.
- Navaneetha R, Saravanan S (2021) Detection of Staphylococcus aureus and antibiotic sensitivity pattern in mastitic cases from Namakkal District. Ind J Dai Sci 74(4): 362-365.
- Mehrotra M, Wang G, Johnson WM (2000) Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J Clin Microbiol 38(3): 1032-1035.
- Kerekes EB, Deák É, Takó M, Tserennadmid R, Petkovits T, et al (2013) Anti‐biofilm forming and anti‐quorum sensing activity of selected essential oils and their main components on food‐related micro‐organisms. J Appl Microbio 115(4): 933-942.
- Benić M, Maćešić N, Cvetnić L, Habrun B, Cvetnić Ž, et al. (2018) Bovine mastitis: a persistent and evolving problem requiring novel approaches for its control-a review. Veterinarski arhiv 88(4): 535-557.
- Cheng WN, Han SG (2020) Bovine mastitis: risk factors, therapeutic strategies, and alternative treatments-A review. Asian-Australas J Anim Sci 33(11): 1699-1713.
- Zaatout N, Ayachi A, Kecha M (2020) Staphylococcus aureus persistence properties associated with bovine mastitis and alternative therapeutic modalities. J Appl Microbio 129(5): 1102-1119.
- Shokohi M, Ahmadizadeh C, Kaveh A (2018) Evaluation of bacterial causes of subclinical mastitis in dairy cattle of negine sabze makoo agro-industrial and animal husbandry complex.
- Mohsenzadeh M, Ghazvini K, Azimian A (2015) Frequency of specific agr groups and antibiotic resistance in Staphylococcus aureus isolated from bovine mastitis in the northeast of Iran. Vet Res Forum 6(4): 295-299.
- Taniguchi T (2021) A 1-Year Investigation of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae Isolated from Bovine Mastitis at a Large-Scale Dairy Farm in Japan. Microb Drug Resist 27(10): 1450-1454.
- Srednik ME, Crespi E, Testorelli MF, Puigdevall T, Pereyra AMD, et al (2019) First isolation of a methicillin-resistant Staphylococcus aureus from bovine mastitis in Argentina. Vet Anim Sci 7: 100043.
- Moslemipur F, Mostafaloo Y, Khanahmadi A (2016) Survey of conformity between organoleptic and microbial culture techniques to diagnose cows’ mastitis and antibiogram test in milk of industrial and traditional herds.
- Tabaee M, Firouzi R, Hamid R (2010) In vitro antibacterial effects of marbofloxacin on microorganisms causing mastitis in cows. J Vet Res 65(1): 51-56.
- Khazaie F, Ahmadi E (2021) Bovine subclinical mastitis-associated methicillin-resistant Staphylococcus aureus, selective genotyping and antimicrobial susceptibility profile of the isolates in Kurdistan province of Iran. Iran J microbiol 13(1): 65-73.
- Hoque M, Das Z, Rahman A, Haider M, Islam M (2018) Molecular characterization of Staphylococcus aureus strains in bovine mastitis milk in Bangladesh. Int J Vet Sci Med 6(1): 53-60.
- Burchacka E, Pstrowska K, Beran E, Fałtynowicz H, Chojnacka K, et al. (2021) Antibacterial Agents Adsorbed on Active Carbon: A New Approach for S aureus and E coli Pathogen Elimination. Pathogens 10(10): 1066.
- Bisht G, Srivastava S, Kulshreshtha R, Sourirajan A, Baumler DJ, et al. (2020) Applications of red pigments from psychrophilic Rhodonellum psychrophilum GL8 in health, food and antimicrobial finishes on textiles. Process Biochemistry 94: 15-29.
- Malik P, Kadam RS, Cheruvu NP, Kompella UB (2012) Hydrophilic prodrug approach for reduced pigment binding and enhanced transscleral retinal delivery of celecoxib. Molecular pharmaceutics 9(3): 605-614.
- Vidya P, Kutty SN, Sebastian CD (2021) Extraction, Characterization and Antimicrobial Properties of Pigments from Yeast, Rhodotorula mucilaginosa Isolated from the Mangrove Sediments of North Kerala, India. Asian J Biol Sci 10(3): 559.
- Ravikumar S, Uma G, Gokulakrishnan R (2016) Antibacterial property of Halobacterial carotenoids against human bacterial pathogens. J Sci Ind Res 75(4): 253-257.
- Boontosaeng T, Nimrat S, Vuthiphandchai V (2016) Pigments production of bacteria isolated from dried seafood and capability to inhibit microbial pathogens. J Environ Sci Toxicol Food Technol 10: 30-34.
- Yolmeh M, Hamedi H, Khomeiri M (2016) Antimicrobial Activity of Pigments Extracted from Rhodotorula glutinis Against Some Bacteria and Fungi. Zahedan J Res Med Sci 18(12): e4954.
- Yolmeh M, Khamiri M, Ghaemi E, Ramezan PS (2018) Antimicrobial Activity of Carotenoid Pigments Extracted from Micrococcus roseus Modares. Journal of Biotechnology 9(4): 565-570.
- Wang W, Zhai Y, Cao L, Tan H, Zhang R (2016) Endophytic bacterial and fungal microbiota in sprouts, roots and stems of rice (Oryza sativa L). Microbiol Res 188: 1-8.
- Wilson C, Lukowicz R, Merchant S, Valquier-Flynn H, Caballero J, et al. (2017) Quantitative and qualitative assessment methods for biofilm growth: A mini-review. Res Rev J Eng Technol 6(4).

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...