Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1217
Review ArticleOpen Access
Psori Silk in Mild to Moderate Psoriasis: Randomized Phase I-II Study Volume 3 - Issue 3
Felix Pavlotsky*, Meir Babaev and Aviv Barzilai
Department of Dermatology, Sackler Faculty of Medicine, Sheba Medical Center, Tel-Hashomer, Israel
Received:July 17, 2021 Published:August 25, 2021
*Corresponding author:Department of Dermatology, Sackler Faculty of Medicine, Sheba Medical Center, Tel-Hashomer, Israel
DOI: 10.32474/OAJCAM.2021.03.000164
Abstract
Objectives: To assess efficacy and safety of Psori Silk in mild to moderate plaque psoriasis.
Material and Methods: Randomized double blind Psori Silk versus vehicle, 12 weeks treatment and 4 weeks follow up study. The primary endpoint was Modified PASI 50 at week 12, while the secondary endpoint included life quality assessment by DLQI.
Results: The Psori Silk group consisted of 23 patients with 34 lesions treated versus 22 and 36 in the vehicle group, respectively.
Modified PASI 50 seen in 59% versus 22.7% of patients in the active and the vehicle group, respectively (p< 0.001). Mean 33% DLQI improvement was observed in 65.2 versus 27.2% (p< 0.001) of active medicine and vehicle group patients, respectively. Mild and temporary discomfort at the application site was observed in 39 and 23% of the active medicine and vehicle group patients, respectively.
Conclusions: Psori Silk appear to be effective and safe treatment for mild to moderate plaque psoriasis. Keywords: plaque psoriasis; natural medicine; Psori Silk; Modified PASI 50; DLQI
Introduction
Around 80% of patients with psoriasis have mild to moderate (localized) disease [1]. For those patients, topical agents are usually the first-line treatment, with corticosteroids most used. Despite their effectiveness, quick relapse and/or rebound are common following discontinuation of those conventional topical treatments [2,3]. Thus, continuous therapy is often needed, however, not feasible due to lack of compliance and/or the possibility of the side effects, especially concerning corticosteroids [4]. Hence, the use of complementary medicine in general and plant-origin topical treatment in particular in up to 69% of the patients [5] is a common practice, and mostly on the their own initiative. However, so far most of the issue related studies have shown a limited efficacy, inconvenience of use, significant adverse events [5-7] and/or a nonrandomized design [8-10]. The purpose of the present study was to examine the efficacy and the safety of the novel Psori Silk multi fruit and vegetable compound in mild to moderate plaque psoriasis.
Material and Methods
The study was approved by the institutional Helsinki board committee. Eligible patients were at least 18 years of age with mild to moderate plaque type psoriasis for at least 6 months before entering the study. The exclusion criteria included treatment by any type of phototherapy, and/or topical anti psoriatic drug 4 weeks prior to entering the study and/or systemic or biologic agents 3 months prior to entering the study. Patients assessed at weeks 0, 2, 4, 8, 12 and 16. At week 0 two representative lesions on different anatomical areas selected. Patients randomized, to either the PSG or the vehicle. To assure double blinding, the PSG and the vehicle gels were indistinguishable regards to texture, smell and visual appearance. Either gel application performed for 12 weeks, followed up by 4 weeks observation without any kind of treatment or lubrication. Patients stopping treatment due to noncompliance during the first two weeks were excluded from the final analysis. The primary efficacy endpoint was Modified PASI (MPASI) 50 at week 12, as previously described [11]. Secondary efficacy endpoints were investigator global assessment (IGA), patient’s global assessment (PGA), and DLQI, all at week 12 [12]. Relapse at the final visit on week 16 was defined, as return of MPASI to the baseline value, while rebound as an increase above baseline MPASI. At week 0 visit, PSG and the vehicle gels monitored for immediate adverse events, while patients applied the assigned products to the chosen lesions and remained in the clinic for 45 minutes. Delayed adverse events were assessed every follow up visit. Statistical intent to treat analysis performed using the R Studio software, version 3.5.1 (2018-07-02) for all the efficacy endpoints. The differences between the PSG and the vehicle groups analyzed using two-tailed one sample T-tests.
Results
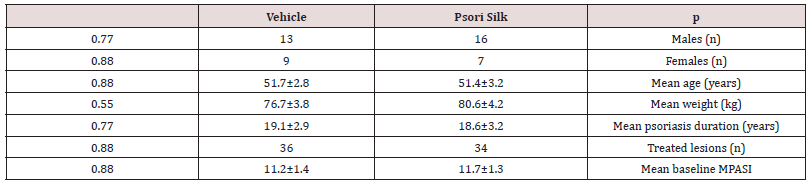
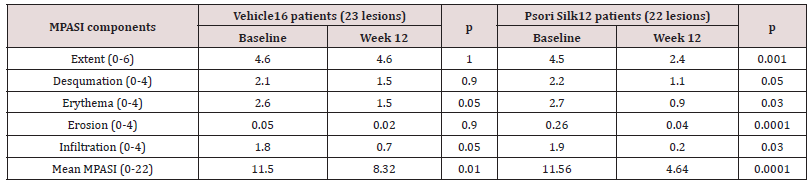
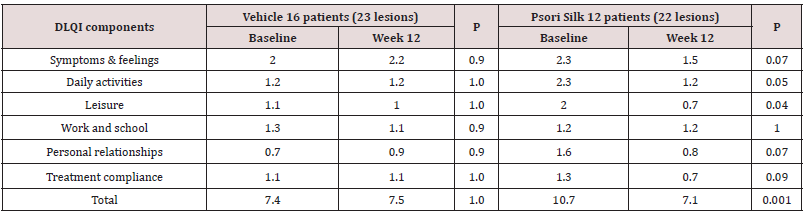
Forty-five included patients’ (70 treated lesions), demographics and mean baseline MPASI by treatment group are shown in Table 1. The main reason for dropout during the first two weeks were the lack of significant improvement in 13 vs 36.3% of the PSG and vehicle patients, respectively. Thus, 28 (62%) patients and 45 lesions (64%) were available for the final analysis. There was no significant difference between the treatment groups regarding age, gender, weight, disease duration and treatment selected lesions number and/or baseline MPASI. MPASI 50 was achieved in 68.2 vs 4.3% of the lesions in PSG and vehicle groups, respectively (P<0.0001). As shown in Table 2, among the individual MPASI ingredients, the lesion’s extent, erythema and desquamation decreased significantly, while Infiltration and erosion showed a pronounced non-significant decrease. All those compared to borderline significant reduction in erythema and infiltration only in the vehicle group. The significant mean MPASI reduction was seen in both groups, however, highly more pronounced in the PSG treated group. At week 12, the IGA score <1 was achieved in 22.8 vs. 8.3% (P≤0.01) in PSG and vehicle treated lesions, respectively. Similarly, at week 12, the PGA score <1 was achieved in 31.4 vs. 11.4% (P≤0.01) in PSG and vehicle treated lesions, respectively. As shown in Table 3, there was a significant improvement in DLQI and most of its individual components in the PSG group only. At week 16, relapse occurred in single lesion in either of the PSG and the vehicle groups. Rebound occurred in a single lesion from the vehicle group, only. Thirty-nine and 23% of the PSG and the vehicle treated patients, respectively, reported mild warm feeling and/or erythema at the application site. The first lasting for 5-10 minutes and the latest resolving up to the second week of treatment in most patients. No other side effects were observed.
Discussion
The two cardinal reasons for patients choosing naturally based topical remedies for plaque psoriasis lesions are the partial efficacy of the traditional ones and the perceived lower risk of the herbal therapies [7,13]. In spite of this public need, the latest have not been successful so far in becoming the front lines choice by most of the physicians or pharmacists. That is mainly due to the fact, that the studies regarding natural plant topical therapies have mostly shown inconclusive/ disappointing results due to limited efficacy, inconvenience of use, adverse events 6-8 and/or non-randomized design [9-11]. All the above, studied the effect of the different single whole plants or their extracts. Thus, the food synergy concept bolstered by the lack of effect of many isolated compounds, as summarized by the American National Institutes of Health [14]. There are studies indicating that combination phytochemical remedies lead to improved additive therapeutic results [15,16] and especially phenols and polyphenols from a combination of the whole (including peel, pulp and seeds) fruits and vegetables [17-20]. Some of those combinations were shown for their various anti-inflammatory actions [17,21,22]. Gloriosa superba and catharanthus roseus extracts have shown a negative synergistic effect on IFN-γ-induced keratin 17 expression in HaCaT human keratinocytes [23]. Taken along the synergistic effects of both soluble free and bound phytochemicals (present only in whole or extract form of fruits and vegetables) on the anti-proliferative activity [19,24] makes those compounds potentially applicable for psoriasis. According to this concept, Psori Silk was composed of two gels (PSG), each containing a specific variety and concentration of 11 different whole (including pulp, peel and seeds) fresh fruits and vegetables. The first, Psori Silk Calm gel intended for soothing/ softening and removing scales, and the second, Psori Silk gel is responsible for the anti-inflammatory action. Due to the low (<500 Da) molecular weight of its mixture fruits and vegetables along the high-water content, the PSG mixture has an easy permeation through the stratum corneum, [25] thickened in the psoriatic process. A preliminary open-label uncontrolled trial of Psori Silk use in 27 plaque psoriasis lesions showed promising results (unpublished observation: E. Ben-Ari, Research Unit, Secret of Youth Ltd. 2015). The purpose of the present study was to evaluate the efficacy and safety of PSG in patients with mild to moderate plaque psoriasis in a randomized double-blind setting. PSG was significantly more effective than the vehicle gel in achieving MPASI 50, along with IGA, PGA and DLQI major improvements. Essentially, only single relapse and/or no rebound shown in our study for four week follow up. The therapy was tolerated extremely well, excluding 3-5 minutes duration of mild warm sensation and slight erythema at the application site during the first 2 weeks of application. The present study, though including relatively small number of patients and treated lesions, shows that in mild to moderate plaque psoriasis, the unique Psori Silk combination of whole fruits and vegetables is effective and safe. Further, larger scale clinical studies are needed, including long-term maintenance use and the possible combination of the later with a short initial topical corticosteroid addition to increase the efficacy and compliance.
References
- Menter A, Gottlieb A, Feldman SR, Abby S van Voorhees, Craig L Leonardi et al. (2008) Guidelines of care for the management of psoriasis and psoriatic arthritis Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 58(5): 826-850.
- Carey W, Glazer S, Gottlieb AB, Mark Lebwohl, Craig Leonardi et al. (2006) Relapse, rebound, and psoriasis adverse events: an advisory group report. J Am Acad Dermatol, 54(4 Suppl 1): S171-181.
- Coondoo A, Phiske M, Verma S, Koushik Lahiri (2014) Side-effects of topical steroids: A long overdue revisit. Indian Dermatol Online J, 5(4): 416-425.
- Lam LH, Sugarman JL (2016) Adrenal suppression with chronic topical corticosteroid use in psoriasis patients. J Drugs Dermatol 15(8): 945-948.
- FuhrmannT, Smith N, Tausk F (2010) Use of complementary and alternative medicine among adults with skin disease: update results from a national survey. J Am Acad Dermatol, 63(3): 1000-1005.
- Deng S, May BH, Zhang AL, C Lu, C C L Xue, et al. (2013) Plant extracts for the topical management of psoriasis: A systematic review and meta-analysis. The British Journal of Dermatology, 169(4): 769-782.
- Farahnik B, Sivamani R, Divya S, Joseph Alban, (2017) Topical botanical agents for the treatment of psoriasis: A systematic review. Am J Clinl Dermatol, 18(4): 451-468.
- Dalaker M, Jacobsen T, Lysvand H, et al. (1999) Expression of the psoriasis-associated antigen, Pso p27 is inhibited by Cyclosporin A. Acta Derm Venereol 79(4): 281-284.
- Smith N, Weymann A, Tausk FA, et al. (2009) Complementary and alternative medicine for psoriasis: a qualitative review of the clinical trial literature. J Am Acad Dermatol 61(5): 841-56.
- Damevska K, Neloska L, Nikolovska S, Gorgi Gocev, Silvia Duma, et al. (2014) Complementary and alternative medicine use among patients with psoriasis. Dermatol Ther 27(5): 281-283.
- Carlin CS, Feldman SR, Krueger JG, Alan Menter, Gerald G Krueger et al. (2004) A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J Am Acad Dermatol 50(6): 859-866.
- Overview of investigator global assessment instruments. Investigator and Patient Global Assessment Measures for Psoriasis Clinical Trials: A Systematic Review on Measurement Properties from the International Dermatology Outcome Measures (IDEOM) Initiative.
- Steele T, Rogers CJ, Jacob SE (2007) Herbal remedies for psoriasis: what are our patients taking? Dermatol Nurs 19(5): 448-463.
- National Institutes of Health (2006) NIH State-of-the-Science Conference Statement on Multivitamin/Mineral Supplements and Chronic Disease Prevention. Ann Intern Med 145: 364-71.
- Genkinger JM, Platz EA, Hoffman SC, George W Comstock, Kathy J Helzlsouer et al. (2004) Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol 160(12): 1223-33.
- Heidemann C, Schulze MB, Franco OH, et al. (2008) Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all-causes in a prospective cohort of women. Circulation 118(3): 230-237.
- Wolfe KL, Kang XM, He XJ, Mei Dong, Qingyuan Zhang et al. (2008) Cellular antioxidant activity of common fruits. J Agric Food Chem 56(18): 8418-8426.
- Song W, Derito CM, Liu KM, Xiangjiu He, Mei Dong et al. (2010) Cellular antioxidant activity of common vegetables. J Agric Food Chem 58(11): 6621-6629.
- Sun J, Chu Y-F, Wu X, Rui Hai Liu (2002) Antioxidant and antiproliferative activities of fruits. J Agric Food Chem 50(25): 7449-7454.
- Eberhardt MV, Lee CY, Liu RH. (2000) Antioxidant activity of fresh apples. Nature 405: 903-904.
- Wilcox IJ, Borradaile NM, Huff MW (1996) Antiatherogenic properties of Narigenin, a citrus flavonoid. Cardiovas Drug Rev 17(2): 160-178.
- Phil-Dong Moon, Choi IN, Kim HM (2011) Naringenin suppresses the production of thymic stromal lymphopoietin through the blockade of RIP2 and caspase-1 signal cascade in mast cells. European Journal of Pharmacology 671(1-3): 128-132.
- Pattarachotanant N, Rakkhitawatthana V, Tencomnao T (2014) Effect of Gloriosa superba and Catharanthus roseus extracts on IFN-γ-Induced Keratin 17 Expression in HaCaT Human Keratinocytes. Evid Based Complement Alternat Med pp. 249367.
- Chu YF, Sun J, Wu X, Rui Hai Liu (2002) Antioxidant and antiproliferative activities of common vegetables. J Agric Food Chem 50(23): 6910-6916.
- Finnin BC, Morgan TM (1999) Transdermal penetration enhancers: application, limitation and potential. J Pharm Sci 88: 955-958.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...