Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1217
Review ArticleOpen Access
Oxalate Degrading Lactic Acid Bacteria in Prevention and Management of Kidney Stone Volume 3 - Issue 5
Deepti Suman1* and Sreeja V2
1,2Dairy Microbiology Department, Kamdhenu University, Gujarat
Received: November 01, 2021 Published: November 24, 2021
*Corresponding author: Deepti Suman, Dairy Microbiology Department, SMC College of Dairy Science, Kamdhenu University, Gandhinagar, Gujarat
DOI: 10.32474/OAJCAM.2021.03.000172
Abstract
Oxalate degrading lactic acid bacteria possessing oxc (Oxalyl-CoA decarboxylase) and frc (Formyl-CoA transferase) genes are now being explored for their application as ingredients for prevention and management of kidney stone. Kidney stones increase the risk of developing chronic kidney disease and is associated with dysbiosis of gut microbiota Vaziri et al. [1]. Probiotics enhances the gut barrier by increasing mucus integrity, epithelial tight junction (ETJ) and epithelial cells survival and also control the overgrowth of pathobionts by reduction of pH and production of antimicrobial peptides (AMPs). Probiotic bacteria belonging to Bifidobacterium and Lactobacillus have been studied for their potential capability of oxalate degradation potential and reported high and efficient degradation of intestinal oxalate and reduces the level of oxaluria in patients with calcium-oxalate kidney stone and mild hyperoxaluria. The key oxalate degrading genes Oxc and frc have been sequenced in Lactobacillus and Bifidobacterium spp. and these strains are commonly used in dairy for probiotic preparation and management of kidney stone and are generally recognised as safe for human consumption. It was demonstrated that oxalate degrading Lactobacillus and Bifidobacterium species possessing probiotic properties and could possibly be used in a prophylactic approach and natural way for prevention and management of kidney stone disease.
Keywords: Oxalate; Lactic acid bacteria; Probiotics; Lactobacilli and Bifidobactrium
Introduction
About 12% of world population is affected by kidney stone during their lifetime and the chance of its recurrence is very high Alelign et al. [2]. In India, approximately 5-7 million patients suffer from stone disease and at least 1/1000 of Indian population needs hospitalization. Kidney stone is associated with chronic kidney disease, bone loss and fractures, increased risk of coronary artery disease, hypertension, type 2 diabetes mellitus, and the metabolic syndrome. Hyperoxaluria increases the risk of calcium -oxalate stone formation and about 80% of kidney stone cases are due to calcium oxalate stone deposition on kidney. Majority of oxalate found in animals originates from ingested oxalate-containing plant material and small portion formed endogenously in the liver via the metabolism of glycine, glyoxylate and ascorbic acid. Human neither possess oxalate degrading enzymes nor oxalate degrading bacteria in their gut and thus oxalate excreted unchanged in the faeces or is absorbed into the urinary tract and due to this the hyper absorption and abnormal synthesis of oxalate leads to kidney stone formation. Many medications and remedies have been used during the past many years and technological advancements have made dramatic improvement in the removal of kidney stones but in spite of this some drawbacks exits like it being too costly for a common man and the chance of its recurrence along with a number of other side effects are very high. Oxalate-degrading bacteria Oxalobacter formigenes possess oxalyl-COA decarboxylase (OXC) and formyl- COA transferase (FRC) enzymes that degrade oxalate into formic acid and CO2 by preventing its absorption in gastrointestinal tract (GIT) of vertebrates, including humans but its survival comes at risk on administration of therapeutic use of antibiotics and other drugs by human and also it lacks the colonization in human gut, further its safety issue and health benefits are not as well established like Lactic Acid Bacteria (LAB). This study was aimed to find the contribution of oxalate degrading lactic acid bacteria (probiotics) in prevention and management of kidney stone. Many in vitro studies have revealed that Lactobacillus spp. possess an excellent oxalate degradation potential and 100% of degradation was observed in lactobacillus acidophilus while Bifidobacterium breve MB 283 de graded 37.8% and Bifidobacterium breve MB 151 degraded only 1% revealing that these degradations are strain specific and may vary from strain to strain. A number of in vivo studies were carried out by many researchers in context to identifying the oxalate degradation potential of lactic acid bacteria and revealed that probiotic fed rat had decreased urinary oxalate excretion indicating a good amount of oxalate degradation in intestine by probiotics. Probiotics containing oxalate degrading lactic acid bacteria can be a good approach in the prevention and management of kidney stone.
Kidney Stone
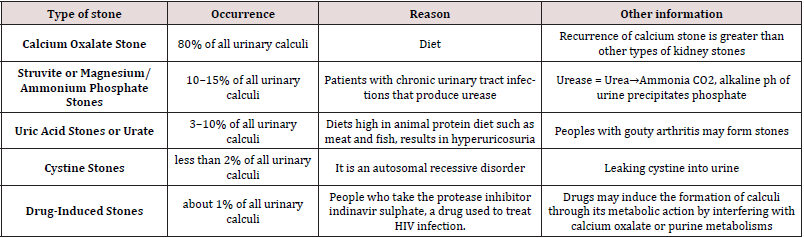
Nephrolithiasis or kidney stone is the presence of renal calculi caused by a disruption in the balance between solubility and precipitation of salts in the urinary tract and in the kidneys Han et al. [3]. It is a complex biological process which involves physicochemical changes and supersaturation of urine and as a result of supersaturation, solutes precipitate in urine leads to nucleation and then crystal concentration is formed. Sequence of events which trigger stone formation are nucleation, growth, aggregation and retention Ratkalkar et al. [4]. Stone is generally an aggregation of solute materials from urine such as calcium, oxalate, phosphate and uric acid.
Types of Kidney Stones
Based on variations in mineral composition and pathogenesis, kidney stones are commonly (Table 1) classified into five types.
Sources of oxalate in human body
Diet
Oxalate usually enters into the body directly from the dietary source and approximately 20-40% of blood oxalate typically derives from dietary (exogeneous) source Hatch [5]. Dietary calcium plays an important role in the oxalate absorption, excess calcium and magnesium in the gut decreases oxalate absorption by binding to oxalate directly, Taylor et al. [6]. while unabsorbed lipids increase the free oxalate concentration by binding to calcium Borghi et al. [7]. Low levels of calcium and magnesium and high-level lipid in the gut all elevate urinary oxalate excretion and the incidence of nephrolithiasis Miller et al. [8].
Liver
A small percentage of oxalate is formed endogenously in the liver via the metabolism of glycine, glyoxylate and ascorbic acid. It is primary source of endogenous oxalate and glyoxylate is the primary immediate precursor of oxalate Huang et al. [9] Glyoxal conversion to glycolate requires glutathione (GSH) where GSH is depleted with increased oxidative stress. High glyoxal concentrations produce reactive oxygen species (ROS) and formaldehyde, they increase cell susceptibility (Table 2) to hydrogen peroxide, and they disrupt the mitochondrial membrane potential, further showcasing its toxic effect.
Table 2: Oxalate degrading lactic acid bacteria commonly inhabiting the gut of humans and animals
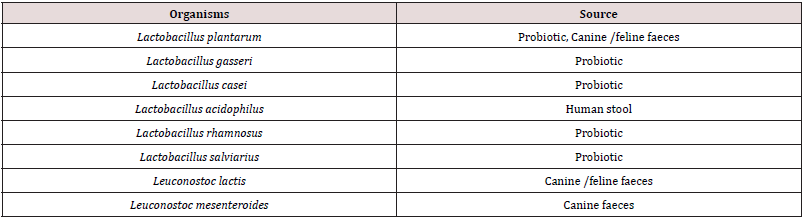
Source: Miller and Dearing 2013
Effect of probiotics on oxalate degradation
Many research workers demonstrated the administration of probiotics and their effect in amelioration of kidney stone. Probiotics can reduce the recurrent calcium-oxalate kidney stone disease by lowering systemic hyperoxaluria Peck et al. [10]. Martins et al 2018 in their study evaluated the effect of probiotic Prato cheese containing Lactobacillus casei 01 (7–8 log CFU/g) in urolithiasis rat model and revealed that PC (Calcium oxalate with probiotic cheese) group presented a significant reduction in the size of the pellets with reduction in potassium, calcium and magnesium excretion further the radiological examination confirmed the role of PC in preventing kidney stone development, which support the PC a superior to the current therapeutics, together with a functional ingredient in nutraceutical applications. Tavasoli et al [11] determined the effect of a probiotic supplement containing native Lactobacillus acidophilus (L. acidophilus) and Bifidobacterium animalis lactis (B. lactis) and observed that only L. acidophilus had a good oxalate degrading activity, in-vitro while no significant effect on urine oxalate was observed in vivo. Al et al 2020 used Drosophila melanogaster model of urolithiasis for evaluation of the therapeutic potential of oxalate-degrading bacteria in calcium oxalate (CaOx) nephrolithiasis. The results demonstrated that Bacillus subtilis 168 (BS168) is a promising candidate based on its preferential growth in high oxalate concentrations, its ability to stably colonize the D. melanogaster. B. subtilis strains used as digestive aids and in fermented foods, these findings suggest that BS168 could represent a novel therapeutic adjunct to reduce the incidence of recurrent CaOx nephrolithiasis in high-risk patients. DONG et al [12] in their study on urolithiasis prevention via oxalate degradation revealed that lactic acid bacteria have the ability to degrade oxalate, which is one of effective ways to prevent calcinmoxalatecalculus in clinical practice. Cho et al. [13] in their assessment of in vitro oxalate degradation by Lactobacillus species cultured from veterinary probiotics revealed that Lactobacillus acidophilus isolates significantly reduced the oxalate concentrations while in vivo studies are needed to determine whether probiotics containing L acidophilus decrease urine oxalate concentrations and reduce risk of kidney stone.
Mechanism of probiotics in treating kidney disease
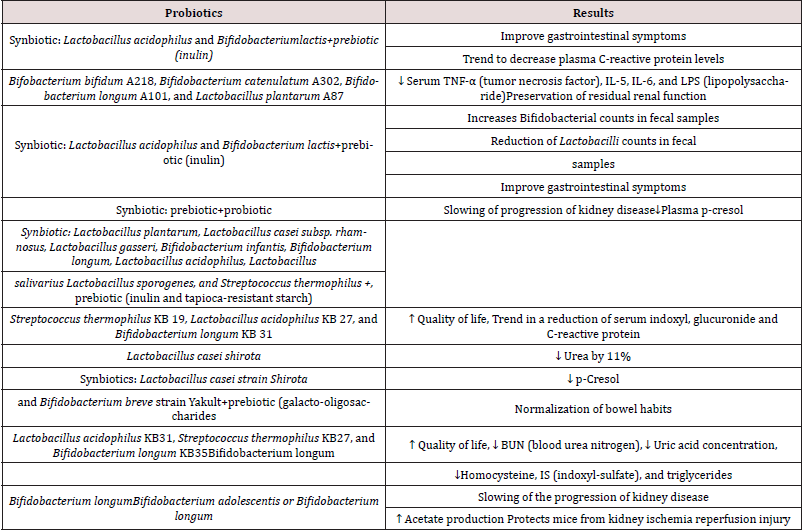
It has been demonstrated that chronic kidney disease (CKD) is associated with dysbiosis of gut microbiota Vaziri et al. [14]. The potential utilization of gut microbiota such as probiotics is an attractive strategy to reduce uremic retention solutes and improve CKD Koppe [15]. Probiotics enhances the gut barrier by increasing mucus integrity, epithelial tight junction (ETJ) and epithelial cells survival and also control the overgrowth of pathobionts by reduction of pH and production of (Table 3) antimicrobial peptides (AMPs). Probiotics could stimulate production of secretory IgA providing additional protection from the luminal microbiota. Probiotics increases the nutrient competition of pathobionts and limits the production of gut-derived uremic toxins Koppe e al. [15]
Isolation and in vitro study of oxalate degrading lactic acid bacteria from different sources
Small population of oxalate degrading lactic acid bacteria is present in the intestine of animal and manipulation of this microbiota definitely helps in the prevention of kidney stone in human. A number of research works were carried out in attempt to identify and evaluate the oxalate degrading lactic acid bacteria inhabiting in animal intestine. Murphy et al. [16] evaluated oxalate degradation potential of Bifidobacteria species and Lactobacillus species isolated from the canine and feline gastrointestinal tract in vitro by growing in oxalate-containing media and their study revealed that the Lactobacillus animalis 223C, Lactobacillus murinus 1222, L. animalis 5323 and L. murinus 3133 showed good oxalate degradation potential. Gomathi et al. [17] screened oxalate degrading Lactic Acid Bacteria (LAB) from human faeces demonstrated the presence of significant population of oxalate degrading LAB in the human intestine. Total 673 strains were isolated from human faeces and fermented foods (appam batter, wheat kali, and curd), among which 251 strains were identified as LAB based on preliminary identification. The presumptive LAB was examined for oxalate utilization using calcium oxalate plate and total of 92 oxalate degrading strains were detected and able to grow and degrade oxalate in the presence of 10 mM potassium oxalate. Significant oxalate degradation was observed in seventeen strains, out of which ten strains utilized more than 50% of oxalate and they reported that the maximum oxalate degradation was detected in L. salivarius AB11 (65.59%) and L. fermentum TY12 (58.5%). In another study by Mahalingam et al. [18] screened oxalate degrading probiotic Lactobacillus from curd sample and instead of potassium oxalate, well dried kidney stone was added in MRS and culture @2% in each vial and incubated at 37⁰C for 1 week and was observed spectroscopically at 600nm and showed maximum percentage of kidney stone degraded (28.6%) on 7th day. Afkari et al. [19] Isolated Lactobacillus spp. from human faeces and were cultured in MRS medium with different concentration 0.5, 0.1, 0.15 and 0.2 percentage of ammonium oxalate, and observed that in concentration of 0.5% and 0.1%, resulted 100% and 90% degradation of oxalate, respectively. Murru et al. [20] screened LAB for their oxalate degrading potential. Cultures were inoculated in MRS-ox, namely MRS modified by sodium oxalate addition, and unmodified MRS and it was observed that around 68% of the strains showed higher growth in MRS broth base than in MRS-ox, but such difference proved to be significant (P<0.05) for 11 strains (Enterococcus spp. 11, 16, 26 and 59, Ec. durans 17, Ec. faecalis 62, Lb. johnsonii La1, St. thermophilus DSM8713 and DSM20617, Lb. reuteri Bio and Lb. casei Lbc496). Similarly, 11 strains showed a better growth in MRS-ox, but the difference was significant for just two strains: Lb. rhamnosus LbGG and St. salivarius DSM20479. The above studies indicate that lactic acid bacteria possess a good oxalate degradation potential being species and strain specific.
In vivo study of oxalate degrading bacteria in rat model
Murphy et al. [21] evaluated oxalate degradation potential of Bifidobacterium and Lactobacillus species isolated from the canine and feline gastrointestinal tract in vivo rat study and showed urinary oxalate levels were significantly reduced (p < 0.05) in animals fed L. animalis 5323 and L. animalis 223C but were unaltered when fed L. murinus 1222, L. murinus 3133 or placebo. Abratt et al. [22] in their Clinical trials resulted the reduced hyperoxaluria through administering Lactobacillus and Bifidobacterium species and showed a promising trend, but they revealed that data need the confirmation through larger scale and well-controlled trials are further required. They emphasized on further investigations to determine whether there is a direct link between the lack of oxalate-degrading bacteria and hyperoxaluria and whether their absence is a risk factor. Taheri et al. [23] in their in vivo ethylene glycol induced rat study used 6 strains of Lactobacillus and 2 Bifidobacterium and revealed a significant reduction of hyperoxaluria, serum creatinine and calcium level were decreased. In the treatment with probiotics, much improvement in the histopathological derangement was observed and showing a remarkable effect as histopathological features of the kidney’s tissue reached up to the normal level which was almost similar to the positive control group.
Effect of oral formulation of oxalate degrading lactic acid bacteria in kidney stone patient
Campieri et al. [24] studied the reduction of oxaluria after an oral course of freeze-dried lactic acid bacteria 8×1011 (L. acidophilus, L. plantarum, L. brevis, S. thermophilus) in idiopathic calcium – oxalate urolithiasis and hyperoxaluria and the treatment resulted in a great reduction of 24-hour excretion of oxalate. A study by Okombo et al. [25] assessed whether a 4-wk daily consumption of a commercially available probiotic (VSL#3®) by 11 healthy volunteers (8 females, 3 males), aged 21-36 y, would decrease oxalate absorption. VSL#3® ingestion has the potential to reduce gastrointestinal oxalate absorption, which could decrease risk of kidney stones and other disorders related to hyperoxaluria. Lieske [26] formulated Oxadrop® specifically for potential treatment of hyperoxaluria and each gram contains 2×1011 bacteria (Lactobacillus acidophilus, L. brevis, Streptococcus thermophilus and Bifidobacterium infantis) and are mixed in a 1:1:4:4 weight and prepared as a granulate. It was observed that patients who received 4 g Oxadrop®, 8 g Oxadrop ®, and 12 g Oxadrop® for 1 month each showed a small effect at 4 and 8 g, with a fall in urine oxalate excretion of about 20–25%. The third month on 12 g of Oxadrop® the urine oxalate excretion was close to baseline. Oxalo Forte is commercially available capsule containing the recombined Lactic Acid Bacteria of Oxalate-Degrading enzyme for treatment of hyper oxaluria.
Genetic modification of Lactic acid bacteria for oxalate degradation
Anbazhagan et al. [27] expressed the heterologous oxalate decarboxylase (OxdC) enzyme in Lactobacillus plantarum and examine its ability to degrade oxalate against hyperoxaluria. The recombinant strain of Lb. plantarum to constitutively overexpress B. subtilis oxalate decarboxylase (oxdC) using a host lactate dehydrogenase promoter (PldhL). They revealed that the recombinant Lb. plantarum was able to degrade more than 90% oxalate compared to 15% by the wild type. In addition, the recombinant strain also had higher tolerance up to 500 mmol l−1 oxalate. In another study Zhao et al. [28] coded the gene of oxalate decarboxylase (ODC) and oxalate oxidase (OxO) into Lactococcus lactis MG1363. The oxalate degradation ability in vitro was evaluated in media with high concentration of oxalate and in vivo study was done in hyperoxaluria rat model with recombinant LAB through oral administration. The result revealed that the recombined LAB with the coding gene of ODC effectively decrease the amount of oxalate in the media and formation of calcium oxalate crystals in kidneys was also inhibited and they also concluded that LAB expressing ODC was more efficient in degradation of oxalate in vitro and in vivo than that expressing OxO. Sasikumar et al. [29] examined the in vivo oxalate degrading ability of genetically engineered Lactobacillus plantarum (L. plantarum) secreting oxalate decarboxylase (OxdC) for prevention of CaOx stone formation in rats. The recombinants strain of L. plantarum that constitutively secreting (WCFS1OxdC) and non-secreting (NC8OxdC) OxdC were developed by using expression vector pSIP401. In vitro condition recombinants L. plantarum express and secretes the functional OxdC and could degrade the oxalate up to 70–77%. In vivo rat study results showed a significant reduction of urinary oxalate, calcium, uric acid, creatinine and serum uric acid, BUN/creatinine ratio. Microscopic observations revealed a high score CaOx crystal in kidneys of groups disease control rat, whereas no crystal in rat treated with recombinant L. plantarum. Many studies have proven that administration of recombinant lactic acid bacteria (LAB) expressing oxalate decarboxylase (OxdC) decreased urinary oxalate excretion and prevented calcium oxalate stone formation Paul et al. [30]. Thus, potential oxalate degrading probiotic recombinant LAB expressing heterologous oxalate decarboxylase could be beneficial for efficiently oxalate degradation in intestinal.
Effect on gut microbial population
Microbiome associated with the healthy urinary tract get altered in urologic disorders. Probiotics, prebiotics, and diet modifications appear to represent an opportunity to regulate the urinary microbiome Aragon et al [31]. Approximately 80% of kidney stones contain oxalate as a primary constituent and diverse oxalate-degrading bacteria exist within the human gut, which may protect against urological disorder. Common shifts in the gut microbiota may facilitate the onset of kidney disease and/or comorbidities Batagello [32]. Manipulation of gut flora with the oxalate degrading lactic acid bacteria may have a positive impact on gut oxalate levels as they are [33] capable of degrading lumen oxalate and reduces the risk of hyperoxaluria. The association between the gut microbiota may facilitate the successful development of bacteriotherapies to inhibit kidney stone disease. In this ecosystem, oxalate-degrading capacities [34] rely on several species, including Lactobacillus, Enterococcus and Clostridium, either with a direct or a permissive role on oxalate degradation.
Conclusion
Kidney stone is a complex disease of world prevalence with high treatment cost and high chance of reoccurrence thus prophylactic approach is one of the best ways in dealing with kidney stone. Oxalate degrading capacity of O. formigenes is well studied but its safety issue and health benefits are not well established as LAB. Manipulation of oxalate degrading lactic acid bacteria in fermented food can be a good approach in prevention and management of kidney stone. Research studies show that oxalate degradation potential of LAB is both species and strain specific. The percent oxalate degradation potential of some of the LAB isolates were promising enough to indicate that they could possibly be used in a probiotic approach for prevention of kidney stone disease.
References
- Vaziri ND, Zhao YY, Pahl MV (2016) Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol Dial Transplant 31(5): 737-746.
- Alelign T, Petros B (2018) Kidney stone disease: an update on current concepts. Adv Urol
- Han H, Segal AM, Seifter JL, Dwyer JT (2015) Nutritional management of kidney stones (nephrolithiasis). Clinical nutrition research. 4(3): 137-52.
- Ratkalkar VN, Kleinman JG (2011) Mechanisms of stone formation. Clinical reviews in bone and mineral metabolism. 9(3-4): 187-197.
- Hatch M (2017) Gut microbiota and oxalate homeostasis. Annals of translational medicine. 5(2): 36.
- Taylor EN, Stampfer MJ, Curhan GC (2004) Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. Journal of the American Society of Nephrology 15(12): 3225-3232.
- Borghi L, Nouvenne A, Meschi T (2010) Probiotics and dietary manipulations in calcium oxalate nephrolithiasis: two sides of the same coin? Kidney international 78(11): 1063-1065.
- Miller AW, Dearing D (2013) The metabolic and ecological interactions of oxalate-degrading bacteria in the mammalian gut. Pathogens 2(4): 636-652.
- Huang HS, Ma MC, Chen CF, Chen J (2003) Lipid peroxidation and its correlations with urinary levels of oxalate, citric acid, and osteopontin in patients with renal calcium oxalate stones. Urology 62(6): 1123-1128.
- Peck AB, Canales BK, Nguyen CQ (2016) Oxalate-degrading microorganisms or oxalate-degrading enzymes: which is the future therapy for enzymatic dissolution of calcium-oxalate uroliths in recurrent stone disease? Urolithiasis 44(1): 45-50.
- Tavasoli S, Jalali S, Naji M, Borumandnia N, Majd GS, et al. (2021) Effect of a Probiotic Supplement Containing Lactobacillus Acidophilus and Bifidobacterium Animalis Lactis on Urine Oxalate in Calcium Stone Formers with Hyperoxaluria: A Randomized, Placebo-controlled, Double-blind and In-vitro Trial. Urology Journal 15: 6789.
- DONG, YUAN JL (2011) Research progress on urolithiasis prevantion via oxalate degradation. Chinese Journal of Microecology 3.
- Cho JG, Gebhart CJ, Furrow E, Lulich JP (2015). Assessment of in vitro oxalate degradation by Lactobacillus species cultured from veterinary probiotics. American journal of veterinary research 76(9): 801-806.
- Vaziri ND, Zhao YY, Pahl MV (2016) Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol Dial Transplant 31(5): 737-746.
- Koppe L, Mafra D, Fouque D (2015) Probiotics and chronic kidney disease. Kidney Int 88(5): 958-966.
- Murphy C, Murphy S, O’Brien F, O’Donoghue M, Boileau T, et al. (2009) Metabolic activity of probiotics—oxalate degradation. Vet Microbiol 136(1-2): 100-107
- Gomathi S, Sasikumar P, Anbazhagan K, Sasikumar S, Kavitha M, et al. (2014). Screening of indigenous oxalate degrading lactic acid bacteria from human faeces and South Indian fermented foods: assessment of probiotic potential. The Scientific World Journal (14): 1-11.
- Mahalingam PU, Rajeshwari P (2014) A study of kidney stone degradation by lactobacillus. Indian J Appl Res 11(4): 47-48.
- Amini H, Jahantigh M, Galavi HR, Abdollahi A, Pirouzi A, et al. (2016). Evaluation of oxalate-degrading activity and molecular Recognition of oxc, frc genes in lactic acid bacterium of inhabit in Human colon. International Journal of Pharmacy and Technology 8(3): 16055-16066.
- Murru N, Blaiotta G, Peruzy MF, Santonicola S, Mercogliano R, et al. (2017) Screening of oxalate degrading lactic acid bacteria of food origin. Ital J Food Saf 6(2).
- Murphy C, Murphy S, O’Brien F, O’Donoghue M, Boileau T, et al. (2009) Metabolic activity of probiotics—oxalate degradation. Vet Microbiol 136(1-2): 100-107.
- Abratt VR, Reid SJ (2010) Oxalate-degrading bacteria of the human gut as probiotics in the management of kidney stone disease. Adv appli microbiol 72: 63-87.
- Taheri H, Miri A, Bokaeian M, Cambyz I, Afkari R et al. (2021) The Evaluation of Lithiasis/Antilithiatic Activity of Oxalate-Degrading Bacteria and Routine Antibiotics. Ann Romanian Soc Cell Biol 25(14): 16514-16522.
- Campieri C, Campieri M, Bertuzzi V, Swennen E, Matteuzzi D, et al. (2001) Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int 60(3): 1097-105.
- Okombo J, Liebman M (2010) Probiotic-induced reduction of gastrointestinal oxalate absorption in healthy subjects. Urological research 38(3): 169-178.
- Lieske JC (2017) Probiotics for prevention of urinary stones. Ann Transl Med 5(2): 29.
- Anbazhagan K, Sasikumar P, Gomathi S, Priya HP, Selvam GS (2013). In vitro degradation of oxalate by recombinant L actobacillus plantarum expressing heterologous oxalate decarboxylase. Journal of applied microbiology 115(3): 880-887.
- Zhao C, Yang H, Zhu X, Li Y, Wang N, et.al. (2018) Oxalate-degrading enzyme recombined lactic acid bacteria strains reduce hyperoxaluria. Urology 113: 253.e1-253.e7.
- Sasikumar P, Gomathi S, Anbazhagan K, Abhishek A, Paul E, et al. (2014). Recombinant Lactobacillus plantarum expressing and secreting heterologous oxalate decarboxylase prevents renal calcium oxalate stone deposition in experimental rats. Journal of biomedical science 21(1): 1-3.
- Paul E, Sasikumar P, Gomathi S, Abhishek A, Selvam GS, et al. (2017) Recombinant LACTIC ACID BACTERIA secreting OxdC as a novel therapeutic tool for the prevention of kidney stone disease. InMultifunctional systems for combined delivery, biosensing and diagnostics pp. 327-345.
- Aragon IM, Herrera-Imbroda B, Queipo-Ortuño MI, Castillo E, Del Moral JS, et al. (2018). The urinary tract microbiome in health and disease. European urology focusses 4(1): 128-38.
- Batagello CA, Monga M, Miller AW (2018). Calcium oxalate urolithiasis: a case of missing microbes?. Journal of endourology 32(11): 995-1005.
- Bangash K, Shigri F, Jamal A, Anwar K (2011) Spectrum of renal stones composition; chemical analysis of renal stones. Int J Pathol 9(2): 63
- Borghi L, Nouvenne A, Meschi T (2010) Probiotics and dietary manipulations in calcium oxalate nephrolithiasis: two sides of the same coin? Kidney international 78(11): 1063-1065.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...