Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1217
Research ArticleOpen Access
High Potency Ginger Extract Reduces Menstrual Discomfort in Healthy Participants with Recurrent Dysmenorrhea Linked to Hypercontractility of the Uterus: a Randomized, Double-Blind, Placebo-Controlled Trial Volume 5 - Issue 1
Somashekara Nirvanashetty* Sanjib Kumar Panda2 and Shavon Jackson Michel3
1CSO, Olene Life Sciences Private Limited, Chennai - 600058, Tamil Nadu, India
2COO, Olene Life Sciences Private Limited, Chennai - 600058, Tamil Nadu, India
3Director of Medical & Scientific Affairs – Dolcas Biotech, LLC 9 Lenel Road, Landing NJ 07850 US
Received: August 29, 2023 Published:September 20, 2023
*Corresponding author: Somashekara Nirvanashetty, CSO, Olene Life Sciences Private Limited, Chennai - 600058, Tamil Nadu, India
DOI: 10.32474/OAJCAM.2023.04.000203
Abstract
Ginger has been widely used for human health yet lack of standardization and poor stability has limited its health applications. This clinical study investigated the efficacy and safety of GINFORT® a ginger extract standardised to >26% gingeroids vs. placebo in primary dysmenorrhea subjects. Fifty female subjects aged between 18 to 35 years with primary dysmenorrhea were enrolled. Participants received either GINFORT® or placebo. The primary endpoint included maximum dysmenorrheic pain VAS score and secondary endpoints included verbal multidimensional scoring system (VMS), evaluation of symptoms reported during menstruation, rescue medication consumption, and subject’s overall satisfaction. There was a significant improvement in the mean VAS score, VMS grade, and symptoms reported during menstruation in GINFORT® group compared to placebo. It was also found to be safe and well tolerated. The findings of this study indicated that GINFORT® is an effective botanical option for women with primary dysmenorrhea.
Keywords: Ginger; Menstrual Discomfort; Primary Dysmenorrhea; VAS; VMS
Introduction
Dysmenorrhea, the medical term describing painful menstruation, is a greater burden than any other gynaecological problem [1]. It is most associated with abdominal/uterine cramps, although other systems and symptoms can be involved. Based on the history and pelvic examination, dysmenorrhea is divided into two subcategories: primary dysmenorrhea (the absence of any identifiable pathologic condition) and secondary dysmenorrhea (the presence of detectable pelvic pathology, such as uterine fibroids, adenomyosis, endometriosis, or pelvic inflammatory disorders) [2]. Primary dysmenorrhea is quite common among women of reproductive age, with an estimated prevalence ranging from 45 to 95% [3]. It has a significant impact on women’s lives, resulting in limited daily activities [4], decreased academic performance [5], and poor quality sleep [6], increased co-morbidity with chronic pelvic pain [3], anxiety, and depression [7]. The impact of dysmenorrhea can even extend beyond the time of menstruation, where it leads to increased pain sensitivity among affected women [8,6]. Researchers have suggested that such increased pain sensitivity may predispose women to developing pain later in life [6]. If the condition is left untreated for a long period of time, it may worsen female infertility [9]. The cause of primary dysmenorrhea is unclear, but uterine thickening and acute uterine contractions caused by excessive prostaglandin (PG) secretion, particularly PGE2 and PGF2 and Leukotrienes are considered the main pathophysiologic features of primary dysmenorrhea [3,10]. Additionally, family history, smoking, excessive bleeding, an irregular menstrual cycle, shorter or longer menstrual intervals, and stress are common risk factors that can intensify symptoms [11]. Most women with primary dysmenorrhea may have additional symptoms such as low back pain, fatigue, nausea, headache, diarrhea, and vomiting [12].
Treating and managing dysmenorrhea not only improves women’s quality of life [13], but it also reduces the risk of female infertility and the development of chronic pain in the future. Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most commonly used first-line pharmacological treatment for dysmenorrhea. However, some dysmenorrhic women are intolerant to NSAIDs [14] and chronic use is known to cause adverse effects including gastrointestinal discomfort, central nervous system symptoms, nephrotoxic and hepatotoxic effects, hematologic abnormalities, bronchospasm, and edema [15]. In these women, hormonal oral contraceptives are often used as second-line therapy, but their prolonged use may be associated with an increased risk of venous thromboembolism16. Furthermore, oral contraceptives are not taken regularly due to concerns about adverse effect [17]. Many women also use non-pharmacological therapies such as heating pads, physical exercise, meditation, aromatic oils, and ginger root tea to relieve menstrual discomfort, although these are often ineffective [3]. Despite the availability of various therapies, there remains a need for safe and effective alternative therapies to relieve menstrual discomfort.
Zingiber officinale (Ginger) is a valuable medicinal plant with a long history of use in various traditional medicines, particularly in Indian and Chinese traditional medicines [18]. The rhizome of Z. officinale has been traditionally used to treat a variety of inflammatory conditions, including pain associated with dysmenorrhea [19]. Gingerol has been found to inhibit prostaglandin (PG) synthesis by inhibiting the activities of cyclooxygenase (COX) and leukotrienes by inhibiting lipoxygenase (LOX) enzymes [18]. However, it is important to note that the effectiveness of herbal extracts depends on the amount of active constituent(s) present [20], which varies depending on the extraction and formulation techniques used [21]. Lack of standardization and stability of ginger extracts are considered as primary reasons for varied results in past clinical trials with ginger extract. GINFORT is a patented and stable high potency ginger extract standardised to>26% gingeroids by HPLC.
GINFORT® is a high potency ginger extract powder standardized to >26% gingeroids. It is developed using a proprietary Aqueosome® technology which converts liquid extracts into powders using very low amount of excipients, which leads products with high actives such as GINFORT®. GINFORT® is the world’s first ginger extract with the highest amounts of gingerols in powder form; currently, there are no technology/products developed and marketed with such a high amount of Gingeroids. The conventional methods for converting liquid oily extracts into powder which requires high amounts of excipients, leads the products with low actives. The standard ginger products which are available in the market contain a maximum of 5% total gingeroids, which is 5 times lesser than the total gingeroids content in GINFORT®. Due to its high content of Gingeroids, GINFORT® has several advantages such as smaller doses (100 mg Vs 500 mg), lesser dose frequency (single dose Vs multiple doses) and hence increased patient compliance.
Aqueosome® technology is green technology which does not require solvents or high power, whereas the conventional methods consume lot of power and organic solvents which adds to greenhouse emissions. The above unique features of Aqueosome® technology led to the development of GINFORT® which solves many of the problems associated with currently available ginger products increasing the patient compliance, efficacy and consistency of benefit. The purpose of this study was to assess the efficacy and safety of the GINFORT® formulation in women with primary dysmenorrhea.
Materials and methods
Study population and sample size
Healthy female subjects with primary dysmenorrhea were screened and enrolled into the study after obtaining informed consent. With a dropout rate of 10%, a convenient sample size of 50 subjects was recruited to ensure at least 44 completers.
Inclusion and exclusion criteria
The study included female subjects aged 18–35 years who had menstrual discomfort in the previous three consecutive months with a maximum dysmenorrhic VAS score of ≥4 and who were diagnosed with primary dysmenorrhea, had a regular menstrual cycle (21–35 days) with a bleeding duration of 3–7 days, and adhere to the study procedure. The exclusion criteria included subjects with secondary dysmenorrhea, endometriosis, metrorrhagia, or oligomenorrhea; lactating and pregnant women or those planning to become pregnant; active NSAID users; and those who had used oral contraceptives or other hormonal medications in the last 3 months. Subjects with a history of cancer, psychological, neurological, haematological, or other clinically significant systemic disorders or known allergies to ginger were also excluded from this study.
Study design
This was a randomized, double-blind, placebo-controlled, parallel-group study. Study subjects were recruited from the clinic of the Chennai Meenakshi Multispecialty Hospital, Chennai, India. After receiving informed consent, subjects were evaluated for eligibility criteria. This 56-day study period included screening visit (V1, day -30); baseline visit (V2, day 1); follow-up visits after the first menstrual cycle (V3, day 28); and after the second menstrual cycle (V4, day 56). At the screening visit all subjects were screened by physical examination, vital signs, demographics, pregnancy test, medical/surgical history, clinical laboratory parameters, medication use, current symptoms, maximum visual analogue scale (VAS) and Verbal Multidimensional Scoring System (VMS) scores. During the baseline visit, eligible participants were assigned in a 1:1 ratio to one of two groups: GINFORT 100 mg or placebo and were instructed to consume one capsule twice daily. A paper-based Case Report Form (CRF) was used to record the data. Figure 1 represents the flow of subjects and the study design.
Efficacy assessment
Primary efficacy measures
The primary endpoint of the study was the mean change from baseline in VAS score. The VAS is a widely used tool to measure pain. VAS is represented by a straight line with extreme limits: from “no pain” and an associated image of a happy face at the left endpoint to “unbearable pain” and an associated image of an unhappy face at the right endpoint. The left endpoint is the minimum pain score of zero, while the right endpoint is the maximum pain score of 10. The pain score was used to classify pain severity by scoring as no pain = 0, mild pain = 1-3, moderate pain = 4-7, and severe pain = 8-10. It was measured at all study visits, and the mean change from baseline was calculated for the entire treatment period.
Secondary efficacy measures
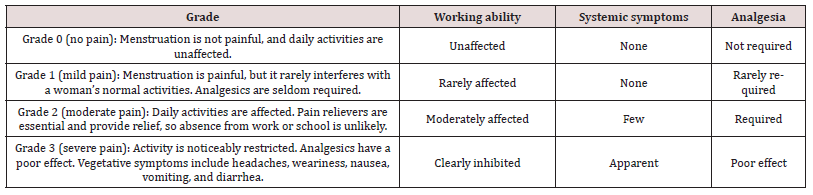
Secondary endpoints included percent change in the VMS, associated symptoms during menstruation, use of rescue medications, and overall subject satisfaction. The VMS grading system uses a scale of zero, one, two, and three to assess working abilities, systemic symptoms, and need for analgesia (Table 1). Various studies have shown its reliability and validity [22-23]. Subjects were instructed to record the severity of the discomfort caused by their menstrual cramps within the first 3 days of menstruation in the baseline cycle, as well as in the first and second treatment follow up cycles according to Table 1.
Most women with primary dysmenorrhea may be accompanied by additional symptoms such as low back pain, fatigue, nausea, headache, diarrhea, and vomiting. If subjects were unable to tolerate the pain, the investigator prescribed rescue medication and the percentage of subjects who used it was calculated. At the final visit, subjects’ overall satisfaction with treatment was assessed using a self-reported improvement score on a numerical rating scale (0 = not satisfied, 1 = mildly satisfied, 2 = mostly satisfied, 3 = completely satisfied).
Safety assessment
Safety parameters were assessed at the baseline visit and at the final visit (day 56). Adverse events and changes in vital signs and laboratory parameters, such as complete blood count, blood biochemistry, lipid profile, renal function, and liver function parameters, were included as safety parameters.
Ethical considerations
The study was approved by the Institutional Ethics Committee (IEC) of Chennai Meenakshi Multispecialty Hospital, Chennai, India. The study has been registered with the Clinical Trials Registry-India (CTRI No.: CTRI/2022/02/039897). The study was carried out in accordance with the Declaration of Helsinki and the International Conference on Principles of Harmonization of Good Clinical Practices (ICH-GCP). The IEC was duly notified of all adverse events and was kept updated on the progress of the trial at regular intervals.
Statistical analyses
An Intention-to-treat (ITT) population was used to assess efficacy. The Mann–Whitney U-test and unpaired t-test were used to detect statistical differences between groups, and Wilcox’s matched-pair rank-sum test for paired data was used to analyse within-group differences, where appropriate. Descriptive statistics were used to describe the demographic and baseline characteristics of the subjects. The SPSS software was used to analyse the data. The accepted level of significance for all analyses was P < 0.05.
Results
Table 3: Comparison of pain intensity score based on VAS from the baseline to first and second menstrual cycle.
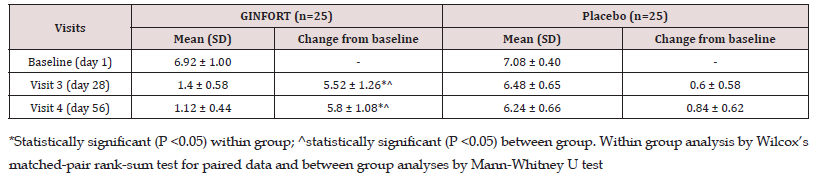
A total of 58 subjects were screened and 50 subjects were enrolled into the study and randomly assigned to either GINFORT 100 mg or placebo group, each with 25 subjects (Figure 1). At the end of the study, 49 subjects completed the study per protocol, and one subject in the placebo group was lost to follow-up at the final visit. Baseline characteristics, including age, weight, height, and BMI for participants in both groups, are provided in Table 2. There was no significant difference observed in any of the baseline characteristics. The primary outcome was the change in maximum pain intensity as measured by VAS after the first (day 28) and second menstrual (day 56) cycles. In the first and second menstrual cycles, the VAS score in the GINFORT group decreased from 6.92±1.0 to 1.4±0.58 and 1.12±0.44, respectively, while in the placebo group it decreased from 7.08±0.40 to 6.48±0.65 and 6.24±0.66, respectively (Table 3). The reduction in maximum pain intensity in the GINFORT group at the first and second menstrual cycles was statistically significant (P < 0.05) compared to the placebo group.
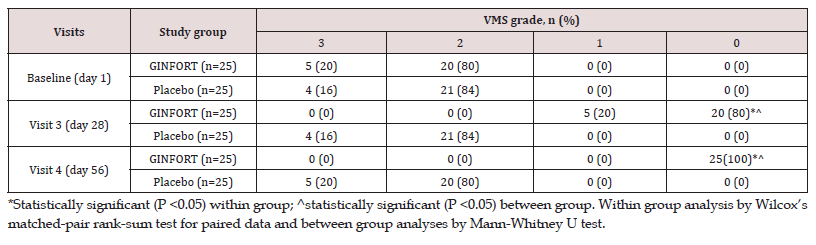
The severity of dysmenorrhic pain was evaluated at baseline and in the first and second menstrual cycles by VMS. In a betweengroup comparison, VMS grade showed a statistically significant improvement (p<0.05) in the GINFORT group compared to placebo at both first and second menstrual cycles. In the GINFORT group, 5 subjects (20%) were with grade 3 and 20 subjects (80%) were with grade 2 dysmenorrhoea severity at baseline, which was changed to 5 subjects (20%) with grade 1 and 20 subjects (80%) with grade 0 dysmenorrhoea severity in first menstrual cycle, but at the end of the second menstrual cycle, all the subjects were with grade 0 dysmenorrhoea severity (Table 4). In the placebo group, the participants were associated with either grade 2 or grade 3 dysmenorrhoea severity at the end of the first and second menstrual cycles. In the placebo group, 16% and 84% of patients had grade 3 and grade 2 dysmenorrhea after the first menstrual cycle, and 20% and 80% at the end of the second menstrual cycle.
Table 4: Comparison of the pain severity based on VMS from the baseline to first and second menstrual cycle.
The prevalence of systemic symptoms was also evaluated at all time points. In the GINFORT group, there was a significant (p<0.05) reduction in the frequency of low back pain, fatigue, and nausea, although there was a significant decrease in symptoms of vomiting after supplementation for two menstrual cycles compared to the placebo group (Table 5). Subjects in either group reported no symptoms of headache or diarrhoea at baseline or at the end of the study (visit 4). In addition, no participants in the GINFORT group required rescue medication, while 2 (8%) subjects in the placebo group required rescue medication at visit 3 and 3 (12%) subjects required rescue medication at visit 4.
The subject’s overall satisfaction level was assessed at the final visit. In the GINFORT group, all subjects reported a score of 3 on a numerical rating scale (0-3), indicating that they were completely satisfied with the treatment. In the placebo group, 11 (45.83%) subjects reported that they were not satisfied with the treatment (score of 0), while 13 (54.17%) subjects were moderately satisfied with the treatment. The overall incidence of adverse events (AEs) in both groups was the same (4%, 1/25 subjects) and all AEs were mild in nature. Only 1 subject (4%) had a cold and cough in the GINFORT group and 1 subject (4%) had a cold only in the placebo group, which were not clinically relevant to the treatments. No serious or severe adverse events were observed in either treatment group. There were no significant changes in laboratory parameters during the study period.
Discussion
Primary dysmenorrhea is a frequent gynaecological problem in young women that causes pain during normal ovulatory cycles with no pathological background [24]. It is closely linked to an increase in PGs and LT production. PGs are synthesized from arachidonic acid by COX and LTs are synthesized by LOX pathways [25]. Arachidonic acid which is released during menstruation in response to falling progesterone level is the common precursor of PGs and LTs which are formed due to the enzymatic action of cyclooxygenase COX-2 and LOX on arachidonic acid. Higher levels of these two pro-inflammatory products of arachidonic acid causes excessive vasoconstriction and uterine smooth muscle contraction leading to pain and other associated symptoms of dysmenorrhea such as nausea, vomiting, and headache [26]. The severity of dysmenorrhoea is correlated with the levels of PGF2 and PGE2, and leukotrienes [26] in menstrual fluid. Drugs that limit PG synthesis can relieve symptoms of dysmenorrhea and are predictively used to treat primary dysmenorrhea. NSAIDs are effective COX-2 inhibitor that reduce PG synthesis and hence alleviates the dysmenorrhic symptoms[27]. Even though mainstream PG inhibitors, such as NSAIDs are highly effective, 10-20% of women with dysmenorrhea are intolerant to these treatments [13].
We can therefore hypothesize that inhibition of the PG pathway alone may be insufficient to relieve menstrual discomfort completely and alternative treatment options with multiple modes of action, which is common with botanicals, could offer more broadreaching benefit. Systematic reviews and meta-analysis of clinical studies have shown the efficacy of herbal products in primary dysmenorrhea [28]. These herbal remedies lessen the symptoms of primary dysmenorrhea by preventing uterine contractions and exerting peripheral analgesic and anti-inflammatory effects by inhibiting PG synthesis [29]. Ginger is a culinary herb with numerous therapeutic applications. Ginger rhizome contains a variety of active compounds, including gingerols, shogaols, gingerdiones, zingerone, and paradol which is responsible for its therapeutic benefits [30]. There are several ways that ginger relieves dysmenorrhea. The active components of ginger, gingerols and gingerdiones, appear to mediate its anti-dysmenorrheal effects. These active components suppress PG production by inhibiting COX-2 and LT production by inhibiting 5-LOX activity 18, which are the two major causative factors for primary dysmenorrhea. This dual inhibitory action of gingeroids makes ginger extract one of the best alternative treatment option in the treatment of primary dysmenorrhea.
Ginger may also inhibit thromboxane production, which activates endorphin receptors and reduces noradrenergic overactivity [8]. The present study revealed that GINFORT supplementation significantly (P<0.05) reduced menstrual discomfort as evidenced by a decrease in VAS score and VMS grade in participants with primary dysmenorrhea as compared to the placebo group. In addition, GINFORT supplementation was observed to minimize the other symptoms of dysmenorrhea (P<0.05), such as nausea, vomiting, fatigue, and low back pain. Over the course of the investigation, none of the GINFORT participants needed rescue medication to manage dysmenorrhic discomfort. Additionally, everyone who received the GINFORT treatment for reducing menstrual discomfort was satisfied with the outcome. This study demonstrated that the GINFORT was not only beneficial in reducing the abdominal cramps and symptoms of primary dysmenorrhea but was also proved safe and free of any adverse effects. Therefore, GINFORT could be an excellent herbal option in the address of symptoms of primary dysmenorrhea, with a better safety profile than NSAIDs. The absence of a dose range evaluation of GINFORT, the inability to measure bleeding volume, the small sample size, and the fact that the study was conducted in a single centre were all considered limitations of the present study. Future research should compare the effects of GINFORT and standard NSAIDs on primary dysmenorrhea.
Conclusion
Finally, the current study revealed that GINFORT (100 mg twice daily) can effectively reduce the severity and intensity of menstrual pain and related symptoms of primary dysmenorrhea. The study also revealed that the recommended dose is well tolerated without experiencing side effects. However, larger-scale clinical studies are required to support the therapeutic feasibility and efficacy of GINFORT as an alternative treatment option for primary dysmenorrhea.
Data Availability Statement
The datasets analyzed during this study will be available from the corresponding author on reasonable request.
Source of Funding
This study was funded and supported by Olene Life Sciences Private Limited, India.
Acknowledgment
The work was supported by Olene Life Sciences Private Limited. The authors are grateful to Dr. R. Vani Raj for managing the clinical studies and providing valuable input.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Ethics approval
This study was conducted in compliance with the ethical principles.
Consent to participate: Not Applicable
Consent for publication: Not Applicable
Code availability: Not Applicable
Authors’ contributions:
Somashekara Nirvanashetty, Ph.D. is into research and development of new products and novel technologies and medical writing.
Mr. Sanjib Kumar Panda, M. Pharm is into designing, planning of clinical studies and data analysis.
References
- Bernardi M, Lazzeri L, Perelli F, Fernando M Reis, Felice Petraglia (2017) Dysmenorrhea and related disorders. F1000 Research 6: 1645.
- Harel Z (2012) Dysmenorrhea in adolescents and young adults: an update on pharmacological treatments and management strategies. Expert Opin Pharmacother 13(15): 2157-2170.
- Iacovides S, Avidon I, Baker FC (2015) What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update 21(6): 762-778.
- Chantler I, Mitchell D, Fuller A (2009)Actigraphy quantifies reduced voluntary physical activity in women with primary dysmenorrhea. J Pain 10(1): 38-46.
- Hailemeskel S, Demissie A, Assefa N (2016) Primary dysmenorrhea magnitude, associated risk factors, and its effect on academic performance: evidence from female university students in Ethiopia. Int J Womens Health 8: 489-496.
- Baker FC, Driver HS, Rogers GG J Paiker, D Mitchell (1999) High nocturnal body temperatures and disturbed sleep-in women with primary dysmenorrhea. Am J Physiol 277(6 Pt 1): E1013-1021.
- Dorn LD, Negriff S, Huang B, et al. (2009) Menstrual symptoms in adolescent girls: association with smoking, depressive symptoms, and anxiety. J Adolesc Health 44(3): 237-243.
- Backon J (1991) Mechanism of analgesic effect of clonidine in the treatment of dysmenorrhea. Med Hypotheses 36(3): 223-224.
- Rabinerson D, Hiersch L, Gabbay-Ben-Ziv R (2018) Dysmenorrhea – Its prevalence, causes, influence on the affected women and possible treatments. Harefuah 157(2): 91-94.
- Pan JC, Tsai YT, Lai JN, et al. (2014) The traditional Chinese medicine prescription pattern of patients with primary dysmenorrhea in Taiwan: a large-scale cross sectional survey. J Ethnopharmacology 152(2): 314-319.
- Ju H, Jones M, Mishra G (2014) The prevalence and risk factors of dysmenorrhea. Epidemiol Rev 36: 104-113.
- Coco AS (1999) Primary dysmenorrhea. Am Fam Physician 60(2): 489-496.
- Proctor M, Farquhar C (2006) Diagnosis and management of dysmenorrhoea. BMJ 332(7550): 1134-1138.
- Dawood MY (1988) Nonsteroidal anti-inflammatory drugs and changing attitudes toward dysmenorrhea. Am J Med 84(5A): 23-29.
- Marjoribanks J, Proctor ML, Farquhar C (2003) Nonsteroidal anti-inflammatory drugs for primary dysmenorrhoea. Cochrane Database Syst Rev (4): CD001751.
- Manzoli L, De Vito C, Marzuillo C, Boccia A, Villari P (2012) Oral contraceptives and venous thromboembolism: a systematic review and meta-analysis. Drug Saf 35: 191- 205.
- Xu L, Xie T, Shen T, Zhang T (2019) Effect of Chinese herbal medicine on primary dysmenorrhea: A protocol for a systematic review and meta-analysis. Medicine (Baltimore) 98(38): e17191.
- Ali BH, Blunden G, Tanira MO, Nemmar A (2008) Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol 46(2): 409-420.
- Crasta S, Fernandes P, Paul S (2019) Ginger Tea on Dysmenorrhoea among Nursing Students. J Health Allied Sci 9(2): 64-75.
- Kesarwani K, Gupta R, Mukerjee A (2013) Bioavailability enhancers of herbal origin: an overview. Asian Pac J Trop Biomed (4): 253-266.
- Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga Latha L (2011) Extraction, isolation and characterization of bioactive compounds from plants' extracts. Afr J Tradit Complement Altern Med 8(1): 1-10.
- Lindh I, Milsom I (2013) The influence of intrauterine contraception on the prevalence and severity of dysmenorrheal: a longitudinal population study. Hum Reprod 28(7): 1953-1960.
- Atallahi M, Akbar SAA, Mojab F, Alavi MH (2014) Effects of wheat germ extract on the severity and systemic symptoms of primary dysmenorrhea: a randomized controlled clinical trial. Iran Red Crescent Med J 16(8): 1-7.
- Kural M, Noor NN, Pandit D, Joshi T, Patil A (2015) Menstrual characteristics and prevalence of dysmenorrhea in college going girls. J Family Med Prim Care 4(3): 426-431.
- Reddy KK, VidyaRajan VK, Gupta A, Aparoy P, Reddanna P (2015) Exploration of binding site pattern in arachidonic acid metabolizing enzymes, Cyclooxygenases and Lipoxygenases. BMC Res Notes 8: 152.
- Guimaraes I, Povoa AM (2020) Primary dysmenorrhea: assessment and treatment. Rev Bras Ginecol Obstet 42(8): 501-507.
- Marjoribanks J, Ayeleke RO, Farquhar C, Proctor M (2015). Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev 2015(7): CD001751.
- Mirabi P, Alamolhoda SH, Esmaeilzadeh S, Mojab F (2014) Effect of medicinal herbs on primary dysmenorrhoea- a systematic review. Iran J Pharm Res 13(3): 757-67.
- Park KS, Park KI, Hwang DS, Lee JM, Jang JB, et al. (2014) A review of in vitro and in vivo studies on the efficacy of herbal medicines for primary dysmenorrhea. Evid Based Complement Alternat Med 296860.
- Rahmani AH, Shabrmi FM, Aly SM (2014) Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. Int J Physiol Pathophysiol Pharmacol 6(2): 125-136.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...