Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1217
Research ArticleOpen Access
High Potency Aged Garlic Extract reduces Cardiovascular Disease Risk Factors in Healthy Participants: A Randomized, Double-Blind, Placebo Controlled Study Volume 5 - Issue 1
Somashekara Nirvanashetty*1 and Sanjib Kumar Panda2
1CSO, Olene Life Sciences Private Limited, Chennai - 600058, Tamil Nadu, India
2COO, Olene Life Sciences Private Limited, Chennai - 600058, Tamil Nadu, India
Received: August 29, 2023 Published:September 20, 2023
*Corresponding author: Somashekara Nirvanashetty, CSO, Olene Life Sciences Private Limited, Chennai - 600058, Tamil Nadu, India
DOI: 10.32474/OAJCAM.2023.04.000202
Abstract
The consumption of aged black garlic and its various preparations has demonstrated potential in conferring cardiovascular health benefits through the mitigation of risk factors associated with cardiovascular disease (CVD). Nevertheless, the outcomes of related studies have exhibited inconsistency, primarily attributable to inadequate standardization, stability concerns, and impractical dosing regimens. In certain instances, the administered doses exceeded practical limits for daily supplementation. This study aimed to evaluate the efficacy of OLNP-15, an extensively potent and stable aged garlic extract meticulously standardized to S-allyl-L-cysteine (SAC), in ameliorating CVD risk factors among healthy participants. Within the framework of a rigorously conducted double-blind, placebo-controlled trial, 56 healthy individuals characterized by borderline CVD risk factor values were randomly assigned to receive either OLNP-15 or a placebo over a 12-week intervention period. OLNP-15 supplementation showed statistically significant reductions (p<0.05) in the mean changes of triglycerides by 19.82 ± 10.34 mg/dL, LDL cholesterol by 19.86 ± 6.75 mg/dL, total cholesterol by 24.00 ± 6.78 mg/dL, fasting blood glucose, and blood pressure. Furthermore, OLNP-15 supplementation led to a notable improvement in HDL cholesterol by 4.82 ± 4.41 mg/dL. The outcomes of this study substantiate that OLNP-15 supplementation represents an efficacious alternative therapeutic approach for both the prevention and treatment of CVDs. Importantly, it was well-tolerated and demonstrated a favourable safety profile, with no discernible adverse effects observed throughout the course of the study.
Keywords: Garlic; cardiovascular; Triglycerides; HDL; SAC
Introduction
Cardiovascular diseases (CVDs) include a spectrum of disorders affecting the heart and blood vessels, including conditions such as coronary heart disease (CHD), cerebrovascular disease, peripheral arterial disease, rheumatic heart disease, congenital heart diseases, deep vein thrombosis, and pulmonary embolism, as defined by the World Health Organization (WHO) [1]. Globally, CVDs are the foremost cause of mortality, responsible for an estimated 17.9 million deaths in 2019, accounting for 32% of all global fatalities, and strikingly, more than 80% of these fatalities occur in low and middle-income countries [2]. The prevalence of cardiovascular disease is surging at an alarming rate [3]. By the year 2030, it is expected that more than 75% of global mortality will be attributed to non-communicable diseases. Notably, CVDs are projected to exceed the cumulative fatalities resulting from infectious diseases (such as HIV/AIDS, tuberculosis, and malaria), maternal and perinatal conditions, and nutritional disorders [4]. Extensive epidemiological research has delineated two categories of risk factors for CVDs: non-modifiable factors, which include age, family history, and gender, and modifiable factors, such as hypertension, diabetes, dyslipidaemia, and obesity. Lifestyle-related risk factors like unhealthy diets, physical inactivity, tobacco use, and excessive alcohol consumption also play a significant role. In the management of CVDs, contemporary strategies primarily emphasize lifestyle adjustments and the targeted control of these modifiable risk factors [5].
Preventive measures assume a central role in the reduction of CVD risk, aiming to effectively manage key risk factors to forestall unfavourable health outcomes. Specifically, in the context of atherosclerosis, numerous therapeutic approaches prioritize the control of hypertension and hyperlipidaemia or seek to modulate hemostasis to avert thrombotic complications. Notably, hypercholesterolemia is a significant contributor to atherosclerosis, and conventional treatments predominantly concentrate on lowering LDL cholesterol levels. Interestingly, despite the extensive range of available pharmaceuticals, only 34% of patients with hypertension achieve comprehensive management [6]. This is often influenced by factors such as the cost and accessibility of antihypertensive agents, as well as concerns regarding their associated side effects [6,7]. Consequently, individuals with hypertension frequently explore Complementary and Alternative Medicine (CAM) remedies, with a notable interest in herbal-based solutions.
Garlic (Allium sativum), a time-honoured ingredient with historical usage spanning more than 5000 years in culinary, dietary, and medicinal contexts, has earned recognition for its diverse applications [8]. Existing literature attests to their beneficial effects on CVD risk factors, with particular emphasis on their potential role in addressing dyslipidaemia. The therapeutic potential of garlic in reducing hypercholesterolemia is well-documented, attributed to its ability to inhibit cholesterol biosynthesis in the liver and protect against the oxidation of low-density lipoproteins [9,10]. Hypertension is also an important cardiovascular disease risk factor. A meta-analysis found that garlic, in three trials, significantly reduced systolic blood pressure (SBP) and, in four trials, lowered diastolic blood pressure (DBP) compared to a placebo [11]. Garlic also exerts a favourable influence on platelet adhesion and aggregation, a potential contributor to cardiovascular disease risk [12]. Multiple studies indicate that garlic achieves these benefits through diverse mechanisms, including reducing serum lipid and blood pressure levels, inhibiting platelet aggregation, and enhancing fibrinolytic and antioxidant activities [13,14]. Research by Rahman and Billington identified several mechanisms through which garlic inhibits platelet aggregation, such as suppressing cyclooxygenase activity, reducing thromboxane A2 formation, inhibiting calcium mobilization in platelets, increasing messengers like cAMP and cGMP within platelets, and boosting the production of plateletderived Nitric Oxide (NO)[15]. Additionally, garlic reduces platelet binding to fibrinogen, resulting in reduced platelet aggregation and enhanced fibrinolytic activity [16].
Accumulated evidence from numerous investigations underscores garlic’s potential in both preventing and managing cardiovascular disorders, especially when taken as a dietary supplement. Nevertheless, a significant challenge in researching garlic’s effects lies in standardizing its preparations, given the volatility and inherent instability of many active garlic constituents. While traditionally, allicin has been considered the primary active compound, recent research indicates that it does not solely account for garlic’s alleged health benefits [17].
S-allyl Cysteine (SAC), a stable water-soluble organosulfur compound found in aged black garlic preparations, has emerged as a potentially significant contributor to the health advantages associated with garlic formulations [17]. In contrast to allicin, SAC has been detected in blood plasma in pharmacokinetic studies involving dogs, rats, and mice [18]. However, discrepancies in the literature persist, primarily attributable to problems related to inadequate standardization, stability concerns, and dosing. There have been instances where study doses were excessively high, making them impractical for daily supplementation, especially among the elderly population. Olene Life Sciences Private Limited has successfully developed a highly concentrated aged black garlic extract formulation, OLNP-15 (Garlzac®) meticulously standardized to contain > 0.5% SAC by HPLC. It is a patent-pending, waterdispersible, and highly stable formulation amicable to multiple dosage forms. This study was undertaken with the objective of evaluating the effectiveness and safety of OLNP-15 in managing cardiovascular risk factors among healthy individuals.
Materials And Methods
Study population and sample size
This study enrolled healthy subjects with cardiovascular disease risk factors based on their lipid profiles and clinical conditions. All eligible participants voluntarily joined the study, and considering a 10% dropout rate, a total of 56 subjects were recruited to ensure a minimum of 50 individuals completed the study.
Inclusion and exclusion criteria
The study enrolled individuals aged 25–65 years who possessed the capacity to comprehend the risks and benefits of the research protocol. Female participants of childbearing potential were required to use an approved method of birth control, while female participants who could not conceive due to either one year of amenorrhea or a history of hysterectomy and/or bilateral oophorectomy were also included. Additionally, participants with serum triglyceride levels between 115 mg/dL and 199 mg/dL, and those willing to provide informed consent and adhere to the study procedures, were included in this study. Exclusion criteria encompassed pregnant or lactating women, individuals with serum glucose levels exceeding 126 mg/dL, those using cholesterollowering medications or supplements, and individuals on blood pressure-lowering medications. Furthermore, participants with abnormal liver or kidney function tests, renal insufficiency, thyroid or other endocrine disorders, or any other condition deemed by the investigator to potentially hinder the subject’s ability to complete the study or its measures, were excluded from participation in this study.
Study design
This study adopted a randomized, double-blind, placebocontrolled, parallel-group design. Participants were recruited via bulletin board announcements at the Chennai Meenakshi Multispecialty Hospital in Chennai, India. Upon obtaining informed consent, potential subjects were assessed for eligibility based on predefined inclusion and exclusion criteria. The study spanned 84 days and involved five key assessment points: the screening visit (V1, day -7), baseline visit (V2, day 0), two follow-up visits (V3, day 28 and V4, day 56), and the end-of-study visit (V5, day 84). During the screening visit, all participants provided written informed consent and underwent a comprehensive screening procedure. Eligible participants were randomly allocated in a 1:1 ratio to one of two groups: either the OLNP-15 500 mg group or the placebo group. Subsequently, all participants received their respective study products and were given instructions to consume one capsule daily for the duration of 84 days. Throughout the study, efficacy parameters, including lipid profiles, blood pressure, fasting blood glucose, adverse events, and rescue medication use, were assessed at screening, follow-up visits (V3 and V4), and the final visit (V5). Additionally, other parameters such as physical examinations, vital signs, and demographic data were evaluated at these time points. Clinical laboratory parameters were specifically assessed at the screening visit and at the final visit. Data collection was conducted using paper-based Case Report Forms (CRFs). For a visual representation of the study flow and subject disposition, refer to Figure 1.
Efficacy assessment
Primary efficacy measures
The primary endpoint of this study was the mean change in serum triglycerides, serum HDL cholesterol, serum LDL cholesterol, and total cholesterol from the baseline measurements. These primary endpoint variables were assessed at all study visits, and the average change from the baseline measurements was calculated for the entire treatment duration.
Secondary efficacy measures
The secondary endpoints encompassed the mean change in fasting blood glucose and blood pressure compared to baseline measurements. These secondary endpoint parameters were assessed during all study visits, and the mean change from baseline was determined for the entire treatment duration.
Safety assessment
Safety assessments were conducted during the baseline visit and the final visit on day 84. These assessments encompassed the occurrence and frequency of adverse events, changes in vital signs, and the analysis of various laboratory parameters, including complete blood count, blood biochemistry, renal function indicators, liver function parameters, and urine analysis.
Ethical considerations
The study protocol was approved by the Institutional Ethics Committee (IEC) of Chennai Meenakshi Multispecialty Hospital, Chennai, India. The study was adhered to the principles outlined in the Declaration of Helsinki and the International Conference on Harmonization of Good Clinical Practices (ICH-GCP). The IEC was promptly informed about all adverse events and regularly updated on the trial’s progress. The study has been registered with the Clinical Trials Registry-India (CTRI/2022/02/039981).
Statistical analyses
An intention-to-treat (ITT) population analysis was employed to evaluate efficacy. Both the primary and secondary endpoints were analyzed using independent sample t-tests for within-group comparisons and repeated measures of ANOVA for between-group comparisons. Descriptive statistics were employed to summarize the subjects’ demographic and baseline characteristics. Data analysis was performed using the SPSS software, and the accepted threshold for statistical significance in all analyses was set at a p-value < 0.05.
Results
A total of 62 subjects were initially screened, out of which 56 subjects met the eligibility criteria and were subsequently enrolled in the study. Six individuals failed to meet the eligibility criteria and were considered screen failures. The enrolled participants were then randomly assigned to either the OLNP-15, 500 mg group or the placebo group, each consisting of 28 individuals (Figure 1). Throughout the study, subjects were closely monitored, and the efficacy endpoints were assessed during the follow-up visits. In total, 53 subjects successfully adhered to the study protocol and completed the study. Nonetheless, within the placebo group, two subjects were lost to follow-up during the final visit, and one participant opted to withdraw from the study. Pertinent baseline characteristics such as age, weight, height, and BMI for participants in both groups are outlined in Table 1. Notably, no significant differences were observed in any of the baseline characteristics.
The primary outcome measured the mean changes in serum triglycerides, serum HDL, serum LDL, and total cholesterol after visit 3 (day 28), visit 4 (day 56), and visit 5 (day 84). There were no significant differences between the groups in these primary endpoint parameters at the baseline. In the OLNP-15 group, the mean serum triglyceride level decreased from a baseline value of 161.64 ± 22.45 mg/dL to 141.82 ± 16.37 mg/dL at visit 5 (p < 0.05). Conversely, in the placebo group, the mean serum triglyceride level decreased from a baseline of 162.14±18.45 mg/dL to 158.18 ± 22.99 mg/dL at visit 5 (Table 2). Both between-group and withingroup analyses revealed that the reduction in serum triglycerides in the OLNP-15 group was statistically significant (p < 0.05) at all post-baseline visits.
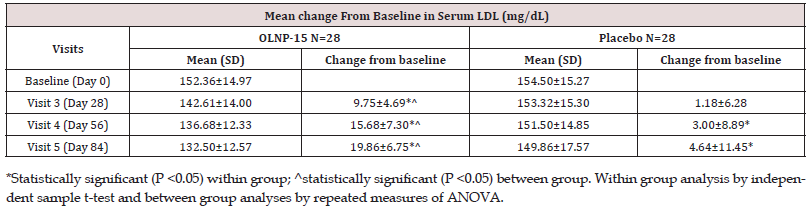
Serum LDL cholesterol levels were assessed at all study visits. In the OLNP-15 group, the baseline serum LDL cholesterol level was 152.36 ± 14.97 mg/dL, and it progressively decreased to 132.50 ± 12.57 mg/dL at visit 5 (p < 0.05). In contrast, in the placebo group, the baseline serum LDL cholesterol level was 154.50 ± 15.27 mg/ dL, which decreased to 149.86 ± 17.57 mg/dL at visit 5 (Table 3). Both between-group and within-group analyses revealed that the reduction in serum LDL cholesterol in the OLNP-15 group was statistically significant (p < 0.05) at all post-baseline visits.
In the OLNP-15 group, serum HDL exhibited a noteworthy improvement, increasing from a baseline value of 41.96 ± 5.71 mg/dL to 46.79 ± 5.36 mg/dL at visit 5. In contrast, in the placebo group, the baseline serum HDL level was 40.82 ± 8.03 mg/dL, and it showed a slight decrease to 40.75 ± 5.47 mg/dL at visit 5. Both between-group and within-group analyses revealed that the increase in serum HDL cholesterol in the OLNP-15 group was statistically significant (p < 0.05) at all post-baseline visits (Table 4).
Total cholesterol exhibited a significant decrease from baseline value of 203.46 ± 11.03 mg/dL to 179.46 ± 11.53 mg/dL at visit 5 in the OLNP-15 group. Conversely, in the placebo group, the baseline total cholesterol level of 205.25 ± 20.19 mg/dL reduced slightly to 203.96 ± 25.09 mg/dL at visit 5 (Table 5). Both between-group and within-group analyses demonstrated that the reduction in serum total cholesterol in the OLNP-15 group was statistically significant (p < 0.05) at all post-baseline visits.
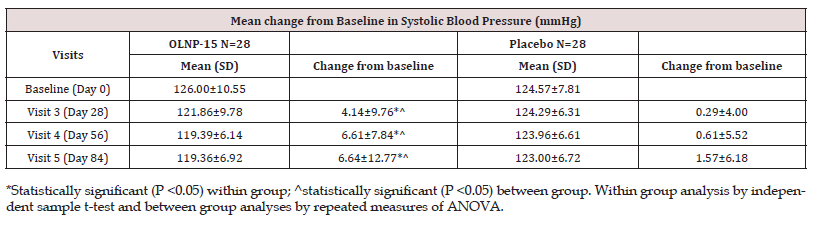
The secondary efficacy endpoints evaluated during the study encompassed the mean changes in blood pressure and fasting blood glucose levels. In the OLNP-15 group, SBP decreased significantly from a baseline value of 126.00 ± 10.55 mmHg to 119.36 ± 6.92 mmHg (p < 0.05) at visit 5, while in the placebo group, it decreased from a baseline value of 124.57 ± 7.81 mmHg to 123.00 ± 6.72 mmHg at visit 5 (Table 6). Notably, the mean change was statistically significant (p < 0.05) in the OLNP-15 group when compared to the placebo. Furthermore, in the OLNP-15 group, DBP exhibited a statistically significant (p < 0.05) reduction at visit 5, decreasing from a baseline value of 84.43 ± 3.51 mmHg to 78.93 ± 1.96 mmHg. In contrast, the placebo group showed a reduction from a baseline value of 81.39 ± 1.87 mmHg to 79.57 ± 1.48 mmHg at visit 5 (Table 7). Importantly, the mean change was statistically significant (p < 0.05) in the OLNP-15 group when compared to the placebo.
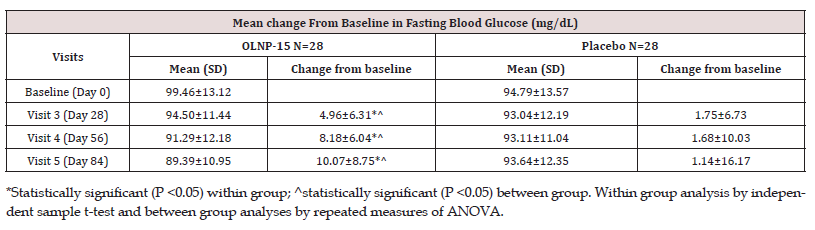
In the OLNP-15 group, there was a statistically significant (p < 0.05) reduction in mean fasting blood glucose from 99.46 ± 13.12 mg/dL at baseline to 89.39 ± 10.95 mg/dL at visit 5 (Table 8). In the placebo group, the mean fasting blood glucose levels remained relatively unchanged, decreasing from 94.79 ± 13.57 mg/ dL at baseline to 93.64 ± 12.35 mg/dL at visit 5. The mean change in fasting blood glucose levels was statistically significant (p < 0.05) in the OLNP-15 group compared to placebo.
The overall incidence of adverse events (AEs) was 14.28% (4 out of 28 subjects) in the OLNP-15 group and 10.71% (3 out of 28 subjects) in the placebo group. Notably, there was no statistically significant difference in the incidence of AEs between the OLNP-15 and placebo groups. Importantly, all AEs were characterized as mild or moderate in severity in both groups, and no clinically relevant AEs were identified. In the OLNP-15 group, the AEs included cold (2 cases, 7.14%), cough (1 case, 3.57%), and toothache (1 case, 3.57%), while in the placebo group, the AEs consisted of fever (1 case, 3.57%) and diarrhea (2 cases, 7.14%). It is worth noting that no serious or severe adverse events were observed in either group, and there were no significant alterations in laboratory parameters during the study period.
Discussion
Cardiovascular diseases (CVDs) represent a significant global health challenge, encompassing a range of conditions affecting the heart and circulatory system[1]. These conditions, including coronary heart disease, hypertension, stroke, and peripheral vascular disease, collectively contribute to substantial morbidity and mortality worldwide[1]. The global health landscape has undergone a significant transformation, shifting away from communicable, maternal, neonatal, and nutritional causes of disease. Instead, there is a growing prominence of non-communicable diseases (NCDs), with CVDs emerging as the foremost contributors to human morbidity and mortality on a global scale [2]. This trend of increasing CVD-related fatalities is expected to persist, with estimates indicating a surpassing 24 million by 2030 [19]. This increase can be attributed in part to changes in lifestyle patterns that are associated with socioeconomic and cultural changes [20]. The high prevalence of CVDs presents a significant challenge for healthcare systems worldwide [21]. As an alternative treatment approach, the utilization of medicinal herbs remains prevalent in addressing various ailments, including CVDs. Notably, there is currently a remarkable surge in the integration of herbal preparations into modern medicinal systems. Several factors contribute to this surge, with cost-effectiveness emerging as a compelling catalyst when compared with conventional modern treatments. Furthermore, there is a widespread belief that herbal remedies represent a safer treatment option.
Garlic (Allium sativum) holds a significant historical and medicinal role in human culture, being an integral part of traditional Indian medicine systems like Ayurveda, Tibbi, and Unani. Its use extends to the management of CVDs, where it is recognized for its multifaceted properties in addressing CVD-related conditions such as hypertension, oxidative stress, inflammation, and hyperlipidemia [12]. Garlic’s effectiveness in managing atherosclerosis and hyperlipidaemia is attributed to its ability to reduce total cholesterol and LDL levels, diminish lipid content in arterial cells, and inhibit the proliferation of vascular smooth muscle cells (VSMCs) [22]. A variety of garlic-based preparations, such as garlic oil, powdered supplements, and pills, are commonly utilized for therapeutic applications. The beneficial effects of garlic are linked to its watersoluble and insoluble sulfur compounds, which regulate metabolic enzymes and demonstrate chelating properties [23].
The accumulation of evidence from diverse studies suggests that garlic holds promise for the prevention and management of cardiovascular ailments and offers potential benefits as a dietary supplement. Nevertheless, a key challenge in investigating garlic’s effects lies in the standardization of its active ingredient within preparations. This is complicated by the volatile and unstable nature of many active components. Notably, allicin, often considered a prominent garlic compound, is itself highly volatile and unstable, rendering its presence uncertain in various garlic preparations. Bioavailability investigations have not identified allicin in either blood or urine, a phenomenon that can be attributed to its inherent instability. Allicin and its derivatives undergo rapid metabolism and excretion, forming transient compounds that decompose into other low molecular weight and volatile sulfur compounds, such as diallyl sulfide (DAS), contributing to the distinctive strong garlic odor and pungent taste [24,20]. A double-blind study involving 42 participants showed no significant alterations in serum HDL cholesterol, triglycerides, blood pressure, and glucose levels during a 12-week supplementation regimen with 900 mg of garlic powder. These inconsistent outcomes noted in clinical investigations are attributed to either the inadequate stability or the absence of product standardization [25].
The SAC, an active compound found in aged black garlic preparations, has been identified as a significant contributor to the health advantages associated with garlic products [17]. SAC, unlike allicin, is a stable and reliable marker for human clinical research involving garlic products. This is because SAC can be identified and accurately quantified in the bloodstream after people orally ingest garlic capsules [17]. Several well-designed and well-controlled human clinical trials provide strong evidence that standardized aged black garlic containing SAC can effectively reduce SBP and DBP, triglycerides, and cholesterol levels [26-28]. However, it’s crucial to emphasize that these favourable outcomes are typically noticed at relatively elevated dosages, often reaching up to 2.4 grams per day. The limited benefits of aged black garlic preparations also seen in some human clinical studies may be due to factors such as decreased stability, lower SAC concentration, or the use of lower therapeutic dosages.
OLNP-15 is a potent aged black garlic extract standardized to contain 0.5% SAC as verified by HPLC analysis. This extract undergoes natural fermentation to enhance the SAC content in the final formulation, achieving an optimal daily dose of 500 mg to maximize health benefits. The current study demonstrates that supplementation with OLNP-15 significantly (p < 0.05) reduced serum triglycerides, serum LDL, and total cholesterol while concurrently improving serum HDL cholesterol levels compared to the placebo group. Moreover, OLNP-15 supplementation was found to significantly (p < 0.05) lower both blood pressure and fasting blood glucose levels. Importantly, this study highlights the safety of OLNP-15 at the dose of 500 mg once daily, with no observed adverse effects. Therefore, OLNP-15 appears to be a viable and safe herbal choice for addressing cardiovascular disease.
Conclusion
In conclusion, this randomized, double-blind, placebo-controlled study demonstrated that supplementation with OLNP-15 at a daily dose of 500 mg over 12 weeks in healthy subjects resulted in statistically significant reductions in cardiovascular risk factors, including triglycerides, total cholesterol, LDL cholesterol, blood pressure, and fasting blood glucose, along with a statistically significant increase in HDL cholesterol. Notably, the study indicated excellent tolerability of OLNP-15, with no participant dropouts. However, further validation of these findings through larger-scale clinical studies is warranted.
Data Availability Statement
The datasets utilized in this study can be obtained from the corresponding author upon reasonable request.
Source of Funding
This study was funded and supported by Olene Life Sciences Private Limited, India.
Acknowledgment
The work was supported by Olene Life Sciences Private Limited. The authors are grateful to Dr. T. Mohana Sundaram for managing the clinical studies and providing valuable input.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Ethics approval: This study was conducted in compliance with the ethical principles.
Consent to participate: Not Applicable
Consent for publication: Not Applicable
Code availability: Not Applicable
Authors’ contributions
Somashekara Nirvanashetty, Ph.D. is into research and development of new products and novel technologies, Mr. Sanjib Kumar Panda, M. Pharm is into designing, planning of clinical studies, medical writing and data analysis.
References
- WHO (2016) Cardiovascular diseases (CVDs).
- WHO (2021) Cardiovascular diseases (CVDs) key facts.
- Gersh BJ, Sliwa K, Mayosi BM, Yusuf S (2010) Novel therapeutic concepts: the epidemic of cardiovascular disease in the developing world: global implications. European Heart Journal 31(6): 642–648.
- Institute of Medicine (US) Committee on Preventing the Global Epidemic of Cardiovascular Disease: (2010) Meeting the Challenges in Developing Countries; Fuster V, Kelly BB, editors. Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health. Washington (DC), National Academies Press (US).
- Fahs I, Khalife Z, Malaeb D, Iskandarani M, Salameh P (2017) The Prevalence and Awareness of Cardiovascular Diseases Risk Factors among the Lebanese Population: A Prospective Study Comparing Urban to Rural Populations. Cardiol Res Pract 2017: 3530902.
- Nguyen Q, Dominguez J, Nguyen L, Gullapalli N (2010) Hypertension management: an update. Am Health Drug Benefits 3(1): 47-56.
- Susalit E, Agus N, Effendi I, Tjandrawinata RR, Nofiarny D, et al. (2011) Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with Captopril. Phytomedicine 18(4):251-258.
- Rivlin RS (2001) Historical perspective on the use of garlic. J Nutr 131(3s): 951S-954S.
- Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A (2004) Interheart Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 364(9438): 937-952.
- Sumiyoshi H (1997) New pharmacological activities of garlic and its constituents. Nihon Yakurigaku Zasshi 110(Suppl 1): 93P-97P.
- Christopher AS, Neil W, Andrew H (1994) A meta-analysis of the effect of garlic on blood pressure. Journal of Hypertension 12(4): 463-468.
- Banerjee SK, Maulik SK (2002) Effect of garlic on cardiovascular disorders: a review. Nutr J 1: 4.
- Arora RC, Arora S, Gupta RK (1981) The long-term use of garlic in ischemic heart disease--an appraisal. Atherosclerosis 40(2): 175-179.
- Khoo YS, Aziz Z (2009) Garlic supplementation and serum cholesterol: a meta-analysis. J Clin Pharm Ther 34(2): 133-145.
- Rahman K, Billington D (2000) Dietary supplementation with aged garlic extract inhibits ADP-induced platelet aggregation in humans. J Nutr 130(11): 2662-2665.
- Rahman K, Lowe GM, Smith S (2016) Aged Garlic Extract Inhibits Human Platelet Aggregation by Altering Intracellular Signaling and Platelet Shape Change. J Nutr 146(2): 410S-415S.
- Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y (2001) Intake of garlic and its bioactive components. J Nutr 131(3s): 955S-62S.
- Nagae S, Ushijima M, Hatono S, Imai J, Kasuga S, et al. (1994) Pharmacokinetics of the garlic compound S-allylcysteine. Planta Med 60(3): 214-217.
- Greenfield DM, Snowden JA (2019) Cardiovascular Diseases and Metabolic Syndrome. In: Carreras E, Dufour C, Mohty M, et al., editors. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies [Internet]. 7th edition. Cham (CH): Springer Chapter 55.
- Kreatsoulas C, Anand SS (2010) The impact of social determinants on cardiovascular disease. Can J Cardiol. 26 Suppl C(Suppl C): 8C-13C.
- Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA (2022) The Global Burden of Cardiovascular Diseases and Risk. J Am Coll Cardiol 80 (25): 2361-2371.
- Sun YE, Wang W, Qin J (2018) Anti-hyperlipidemia of garlic by reducing the level of total cholesterol and low-density lipoprotein: A meta-analysis. Medicine (Baltimore) 97(18): e0255.
- Subramanian MS, Nandagopal MS G, Amin Nordin S, Thilakavathy K, Joseph N (2020) Prevailing Knowledge on the Bioavailability and Biological Activities of Sulphur Compounds from Alliums: A Potential Drug Candidate. Molecules 25(18): 4111.
- Lawson LD, Ransom DK, Hughes BG (1992) Inhibition of whole blood platelet-aggregation by compounds in garlic clove extracts and commercial garlic products. Thromb Res 65 (2): 141-56.
- Cardelle-Cobas A, Soria AC, Corzo-Martínez M, Villamiel M (2019) A Comprehensive Survey of Garlic Functionality. Pacurar M, Krejci G (eds.), In: Garlic Consumption and Health. Nova Science Publishers Inc., 2010.
- Vlachojannis C, Schoenenberger AW, Erne P, Chrubasik-Hausmann S(2019) Preliminary evidence of the clinical effectiveness of odourless garlic. Phyther Res 33(9): 2179-2191.
- Valls RM, Companys J, Calderón-Pérez L, Salamanca P, Pla-Pagà L, e al. (2022) Effects of an Optimized Aged Garlic Extract on Cardiovascular Disease Risk Factors in Moderate Hypercholesterolemic Subjects: A Randomized, Crossover, Double-Blind, Sustainedand Controlled Study. Nutrients 14(3): 405.
- Villaño D, Marhuenda J, Arcusa R, Moreno-Rojas JM, Cerdá B, et al. (2023) Effect of Black Garlic Consumption on Endothelial Function and Lipid Profile: A Before-and-After Study in Hypercholesterolemic and Non-Hypercholesterolemic Subjects. Nutrients 15(14): 3138.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...