
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1217
Case ReportOpen Access
A minimally invasive supraorbital keyhole approach through superciliary arch for giant olfactory Groove angiopericytoma: case report Volume 2 - Issue 5
Pengxiang Yan, MD1#, Weiliang Zhao, MD2#, Sen Xie, MD2, Yanhui Sun, MD1 and Penglian Wang, MD, PhD3*
- 1Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2Department of Neurosurgery, The Third Medical Centre, Chinese PLA (People’s Liberation Army) General Hospital, Beijing, China
- 3Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
Received: October 12, 2020; Published: November 02, 2020
*Corresponding author: Professor Penglian Wang, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University,
Beijing, China, Tel: +86-10-59976505, Fax: +86-10-59976641
#The first two authors contributed equally to this work.
Abstract
We sought to demonstrate a giant hemangiopericytoma within the olfactory groove resected by a minimally invasive supraorbital keyhole approach through superciliary arch, which is commonly used to remove relatively small meningioma, pituitary adenoma and craniopharyngioma. A 50-year-old female presented with a 1-year history of progressive headaches, anosmia, hyposmia, and visual deterioration. Anosmia and visual impairment were found by physical examination. The magnetic resonance imaging (MRI) scan demonstrated a large irregularly shaped intra-dural mass at bottom of right frontal lobe. A minimally invasive supraorbital keyhole approach through superciliary arch is performed for resecting this giant tumor. Surgical complications and degree of resection were recorded to evaluate the efficacy of this surgical method. Histological examination confirmed a diagnosis of hemangiopericytoma originating from the olfactory groove. Gross total resection of the intracranial hemangiopericytoma was possible with minimal brain retraction. Simpson grade I was achieved, and there were no presentation of new neurologic deficits, postoperative hematomas, and cerebrospinal fluid leakage in patient. We suggested that it is worthwhile a try to remove giant olfactory groove hemangiopericytoma by the minimally invasive supraorbital keyhole approach through superciliary arch, allowing for minimal damage of normal brain parenchyma, and improving prognosis.
Keywords: Hemangiopericytoma; Invasive; Supraorbital keyhole approach; Superciliary arch; Olfactory groove
Abbrivations: CSF: Cerebro Spinal Fluid; CT: Computed Tomography; MRI: Magnetic Resonance image
Introduction
Hemangiopericytoma of the central nervous system, also known as vascular pericytes tumor, which is a rare mesenchymal tumor which derive from malignant transformation of pericytes. Hemangiopericytomas comprise less than 1% of all the intracranial tumors [1]. Because of hemangiopericytomas with abundant blood supply, the surgical removal can carry significant risk. The Bi-coronal frontal approach, frontolateral approach, unilateral tailored fronto-orbital approach and invasive interhemispheric approach are usually used for hemangiopericytomas located in the olfactory groove, with good outcomes [2-8]. The supraorbital keyhole approach through superciliary arch incision for anterior midline skull base lesions has become increasingly, especially for relatively small meningiomas [9-19]. However, few articles have addressed the utility of the superciliary arch incision in the treatment of intracranial hemangiopericytomas. Therefore, we performed a minimally invasive supraorbital keyhole approach through superciliary arch for microsurgical resection of intracranial hemangiopericytoma. The idea behind this technique is to achieve an effective surgical resection with less brain damage.
Case Description
A 50-year-old female presented with a 1-year history of progressive headaches, anosmia, hyposmia, and visual deterioration. A comprehensive review of the systems and the physical examination were negative, except anosmia and visual impairment. The magnetic resonance imaging (MRI) scan demonstrated an irregularly shaped 6.1× 5.2 × 5.2cm intra-dural mass at bottom of right frontal lobe, which was enhanced by gadolinium contrast with the cork-screw type of intra-tumoral vessels, highly suspicious of hemangiopericytoma or meningiomas arising from the olfactory groove Figure 1 A-C. The skull was free of mass invasion.
Figure 1: Preoperative MR images.
A large intra-dural mass at bottom of right frontal lobe was heterogeneously enhanced T1-weighted axial, coronal, and sagittal
contrast MRI, with the cork-screw type of intra-tumoral vessels (A, B and C).
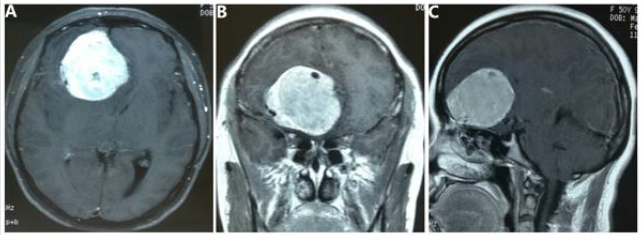
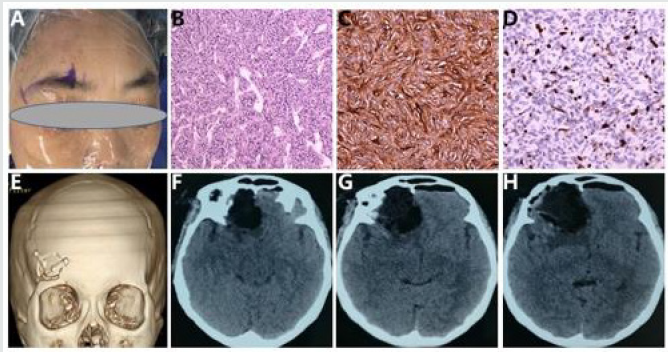
Figure 2 Intraoperative photographs, histological examination, and postoperative CT images.
The minimally invasive supraorbital keyhole approach through superciliary arch was used to remove the tumor (A).
Hematoxylin and eosin staining showed that tumor cells arrayed closely with an average nuclear division of about 3/10HPF,
and thin-walled blood vessels scattered among tumor cells (original magnification x100) (B). Most tumor cells are strongly
positive for CD 34 (C) and about 67% positive for Ki-67 (D) on immunohistochemical staining (original magnification x200).
The postoperative computed tomography (CT) confirmed the minimal eyebrow incision (E), and the complete removal of the
tumor at six hours following operation (Figure F, G and H).
To remove mass and reduce brain damage, we decided using
a minimally invasive supraorbital keyhole approach through
superciliary arch Figure 2A. After general anesthesia, the patient
is placed supine on the operating table with their head fixed in a
three-pin Mayfield head holder. The head of patient is elevated
until exceeding the level of the thorax, and retroflexed 15-30°.
The skin is incised laterally from the supraorbital incisura within
the eyebrow in a lateral-to-medial direction Figure 2A. Following
the skin incision, the subcutaneous dissection is continued in the
frontal direction to achieve optimal exposure of the frontolateral
supraorbital area. The frontalis muscle is then cut with a monopolar
knife parallel to the orbital rim in a medial-to-lateral direction. A
single hole is made using a highspeed drill at the level of the frontal
skull base. A minimal craniotomy is carried on using a high-speed
craniotome, which cut the surface within a size of approximately
25×20mm. The dura is opened in a simple “C” shape and retracted
in a basal direction. With careful mobilization of the frontal lobe,
the deep subarachnoid cisterns could be reached without using
an elevator. After release of cerebrospinal fluid (CSF), the tumor
is approached in a retractor-free manner and is carefully removed
under microscopic visualization, using cottonoids to protect brain
during dissection. At the same time, the ethmoidal arteries are
coagulated for reducing bleeding during the dissection. Careful
dissection between the tumor and neurovascular structures usually
requires a great amount of time and effort. A gross total resection
of giant hemangiopericytoma was carried out with minimal brain
retraction and minimal skull lesion Figure 2E. After tumor was
removed with Simpson grade I, the dura was reapproximated with
watertight closure and dura anchoring, as well as biologic glue
applied. The bone flap is fixed back in place by titanium screw
Figure 2E. Finally, the wound is closed with 2-0 Vycril and 3-0 Nylon.
The histopathologic result revealed that that tumor cells
arrayed closely with an average nuclear division of about
3/10HPF, and thin-walled blood vessels scattered among tumor
cells Figure 2B. The tumor cells were positive for CD34 and Ki67
by immunohistochemical staining Figure 2C & D. There were no
surgical complications. Postoperatively, the patient did not present
any increased neurological impairment except anosmia and visual
impairment. The postoperative computed tomography (CT)
confirmed the complete removal of the tumor at six hours following
operation (Figure 2 F-H).
At discharge, the patient was ambulated independently without
headache. She was referred to a radiation oncologist for adjuvant
therapy. Her vision significantly improved, and her olfactory function
were slightly improved at 6 months after operation. The scar within
the patient’s right eyebrow is barely visible. The MRI scan also
confirmed the complete removal of the hemangiopericytoma at six
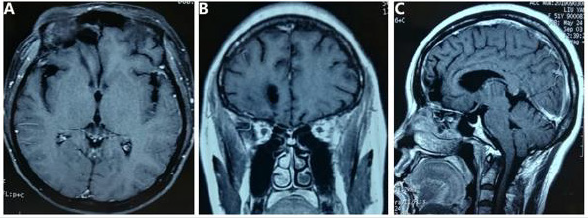
months postoperatively (Figure 3 A-C).
Figure 3 Postoperative MR images.
Magnetic Resonance scan (6 months postoperatively) revealed a complete removal of the tumor on T1-weighted axial, coronal,
and sagittal contrast images (A, B and C).
Discussion
Intracranial hemangiopericytomas are very rare vascularized
mesenchymal tumors with an incidence of less than 1% [1],
and they represented 2.5% of all meningeal tumors [20].
Hemangiopericytomas are highly vascular tumors, which derived
from pericytes around capillaries and postcapillary venules. Up to
now, only one case of Hemangiopericytoma derived from olfactory
groove was previously reported [2]. We added another case of giant
hemangiopericytoma originating from olfactory groove, which was
removed by a minimally invasive supraorbital keyhole approach
through superciliary arch.
Though there was no specificity in brain CT and MRI for
most tumors originated from the anterior skull base [21-23], the
origination of huge tumors could be presented by MRI [24]. The
diagnosis of tumor originating from olfactory groove was highly
recommended by MRI in this patient. However, it is extremely
important distinguishing intracranial hemangiopericytoma from
meningioma in the anterior skull base because surgical resection of intracranial hemangiopericytoma will take a more bleeding risk
than resection of meningioma. The previous brain MRI studies
demonstrated that the cork-screw type of intra-tumoral vessels
suggested intracranial hemangiopericytoma [21,24], whilst the
spoke-wheel type of intra-tumoral vessels seemed more like
meningioma25. Presenting the cork-screw type of intra-tumoral
vessels in MRI Figure 1 of this patient also reasonably suggested
the diagnosis of hemangiopericytoma, hinting neurosurgeon need
consciously consider approaches and prevention postoperative
complications.
Traditional approaches used to resect tumors of anterior skull
base, especially for larger tumors, include subfrontal unilateral
craniotomy, subfrontal bilateral craniotomy, interhemispheric
approach, bifrontal approach,pterional craniotomy, and
craniotomy with adjuvant radiotherapy [3,4,6,8,26-29]. The
supraorbital keyhole craniotomy has been developed for reducing
skin incision, bony exposure, soft-tissue damage, and improving
prognosis of patients with frontal base tumors by lots of surgeons
[9-19,30-33]. Its safety and efficacy are comparable to those of
conventional craniotomies for small size intra-axial lesions (such
as gliomas, metastatic carcinomas, radiation necroses, intracranial
contusion hematomas, and cavernomas), and small size extraaxial
lesions (such as meningioma, craniopharyngioma, pituitary
adenomas, aneurysms) in the anterior or middle cranial fossa [9-
19,30-33]. Giant tumors of anterior skull base favor to invade nerves,
vessels, cerebral dural sinuses and other critical neuroanatomical
areas surrounding tumors [5].
In order to remove these giant tumors, neurosurgeons usually
choose the bilateral subfrontal craniotomy and the unilateral
subfrontal craniotomy with/without adjuvant radiotherapy [2-5].
The previous studies also reported that surgeons tried to grossly
resect giant olfactory groove meningiomas by unilateral tailored
fronto-orbital approach and invasive interhemispheric approach
[7,8]. Up to now, only one case of intracranial hemangiopericytoma
arising from the olfactory groove was gross totally resected by Bicoronal
frontal craniotomy [2]. However, these approaches usually
require more operative time, and need a large bony defects and
long skin incisions, which might be relevant with poor prognoses.
We first tried to remove giant olfactory Groove angiopericytoma
through the minimally invasive supraorbital keyhole approach
through superciliary arch. Postoperative complications of this
minimally invasive craniotomy usually include leakage of CSF,
supraorbital hypesthesia, palsy of the frontal branch of the facial
nerve, wound healing disturbance or wound infection, blindness
and hemiplegia [33]. Except for supraorbital hypesthesia, other
postoperative complications did not occur in our patient with
this minimally invasive supraorbital keyhole approach through
superciliary arch.
We believe that this minimally invasive supraorbital
keyhole approach through superciliary arch is a good choice for
hemangiopericytoma in the olfactory groove due to the good
outcome on the postoperative recovery. This approach has some
advantages: a minimal skin incision, minimal bony exposure,
minimal soft-tissue trauma, and less damage to olfactory nerve.
Gross total resection is perfectly possible with minimal brain
retraction. Concentration, familiar with anatomical structure and
reliable hemostasis technology are needed to resect tumor in
order to avoid injuring intracranial neurovascular structures using
this minimally invasive supraorbital keyhole craniotomy through
superciliary arch.
Conclusion
Intracranial hemangiopericytomas are rare tumor which are highly vascular tumors. The surgical removal can carry significant risk. In this case, the minimally invasive supraorbital keyhole approach through superciliary arch provides an effective and safe route for giant hemangiopericytoma in the olfactory groove with little or no need for brain retraction. Base on familiar with anatomical structure and reliable hemostasis technology, total resection of the tumor with abundant blood supply in the anterior skull base, can be achieved with minimal invasive supraorbital keyhole approach through superciliary arch.
Acknowledgment
The authors would like to thank the patient who consented to participating in this study.
Funding
None.
Disclosure
The authors have no conflicts of interest to disclose.
References
- Schirmer CM, Heilman CB (2011) Hemangiopericytomas of the skull base. Neurosurg Focus 30(5): E10.
- Gupta R, Moore JM, Miller K, Harsh GR (2017) Hemangiopericytoma in the Olfactory Groove: A Rare and Unusual Presentation. Cureus 9(11): e1875.
- Jian BJ, Han SJ, Yang I, Waldron JS, Tihan T, et al. (2010) Surgical resection and adjuvant radiotherapy for a large pineal hemangiopericytoma. J Clin Neurosci 17(9):1209-1211.
- Gazzeri R, Galarza M, Gazzeri G (2008) Giant olfactory groove meningioma: ophthalmological and cognitive outcome after bifrontal microsurgical approach. Acta Neurochir (Wien) 150(11): 1117-1125.
- Martin J Rutkowski, Brian J Jian, Orin Bloch, Cheng Chen, Michael E Sughrue, et al. (2012) Intracranial hemangiopericytoma: clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer 118(6): 1628-1636.
- Nakamura M, Struck M, Roser F, Vorkapic P, Samii M (2008) Olfactory groove meningiomas: clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery 62(6 Suppl 3): 1224-1232.
- Downes AE, Freeman JL, Ormond DR, Lillehei KO, Youssef AS (2015) Unilateral Tailored Fronto-Orbital Approach for Giant Olfactory Groove Meningiomas: Technical Nuances. World Neurosurg 84(4): 1166-1173.
- Liborio Dos Santos AR, Calfat Maldaun MV, Gripp DA, Watanabe J, Fujiki RH, et al. (2018) Minimally Invasive Interhemispheric Approach for Giant Olfactory Groove Meningioma: Technical Note. World Neurosurg 120: 316-319.
- Ya-Jui Lin, Ko-Ting Chen, Cheng-Chi Lee, Cheng-Hong Toh, Tai-Wei Erich Wu, et al. (2018) Anterior Skull Base Tumor Resection by Transciliary Supraorbital Keyhole Craniotomy: A Single Institutional Experience. World Neurosurg 111: e863-e870.
- Reisch R, Perneczky A (2005) Ten-year experience with the supraorbital subfrontal approach through an eyebrow skin incision. Neurosurgery 57(4 Suppl): 242-255.
- Wiedemayer H, Sandalcioglu IE, Wiedemayer H, Stolke D (2004) The supraorbital keyhole approach via an eyebrow incision for resection of tumors around the sella and the anterior skull base. Minim Invasive Neurosurg 47(4): 221-225.
- Czirjak S, Nyary I, Futo J, Szeifert GT (2002) Bilateral supraorbital keyhole approach for multiple aneurysms via superciliary skin incisions. Surg Neurol 57(5): 314-323.
- Fatemi N, Dusick JR, de Paiva Neto MA, Malkasian D, Kelly DF (2009) Endonasal versus supraorbital keyhole removal of craniopharyngiomas and tuberculum sellae meningiomas. Neurosurgery 64(5 Suppl 2): 269-284.
- Chen HC, Tzaan WC (2010) Microsurgical supraorbital keyhole approach to the anterior cranial base. J Clin Neurosci 17(12): 1510-1514.
- Berhouma M, Jacquesson T, Jouanneau E (2011) The fully endoscopic supraorbital trans-eyebrow keyhole approach to the anterior and middle skull base. Acta Neurochir (Wien) 153(10): 1949-1954.
- Hong WC, Tsai JC, Chang SD, Sorger JM (2013) Robotic skull base surgery via supraorbital keyhole approach: a cadaveric study. Neurosurgery 72 Suppl 1:33-38.
- Alhadi Igressa, Ioannis Pechlivanis, Friedrich Weber, Mehran Mahvash, Ali Ayyad, et al. (2015) Endoscope-assisted keyhole surgery via an eyebrow incision for removal of large meningiomas of the anterior and middle cranial fossa. Clin Neurol Neurosurg 129: 27-33.
- Ditzel Filho LF, McLaughlin N, Bresson D, Solari D, Kassam AB, et al. (2014) Supraorbital eyebrow craniotomy for removal of intraaxial frontal brain tumors: a technical note. World Neurosurg 81(2): 348-356.
- Ormond DR, Hadjipanayis CG (2013) The Supraorbital Keyhole Craniotomy through an Eyebrow Incision: Its Origins and Evolution. Minim Invasive Surg 2013: 296469.
- Kim YJ, Park JH, Kim YI, Jeun SS (2015) Treatment Strategy of Intracranial Hemangiopericytoma. Brain Tumor Res Treat 3(2): 68-74.
- El-Ali AM, Agarwal V, Thomas A, Hamilton RL, Filippi CG (2019) Clinical metric for differentiating intracranial hemangiopericytomas from meningiomas using diffusion weighted MRI. Clin Imaging 54:1-5.
- Nobuhiko Arai, Katsuhiro Mizutani, Satoshi Takahashi, Yukina Morimoto, Takenori Akiyama, et al. (2018) Preoperative Assessment of Pathologic Subtypes of Meningioma and Solitary Fibrous Tumor/Hemangiopericytoma Using Dynamic Computed Tomography: A Clinical Research Study. World Neurosurg 115: e676-e680.
- Tanhui Chen, Bingqing Jiang, Yingyan Zheng, Dejun She, Hua Zhang, et al. (2020) Differentiating intracranial solitary fibrous tumor/hemangiopericytoma from meningioma using diffusion-weighted imaging and susceptibility-weighted imaging. Neuroradiology 62(2): 175-184.
- Pang H, Yao Z, Ren Y, Liu G, Zhang J, et al. (2015) Morphologic patterns and imaging features of intracranial hemangiopericytomas: a retrospective analysis. Onco Targets Ther 8: 2169-2178.
- Watts J, Box G, Galvin A, Brotchie P, Trost N, et al. (2014) Magnetic resonance imaging of meningiomas: a pictorial review. Insights Imaging 5(1): 113-122.
- Avinash KS, Thakar S, Ghosal N, Hegde AS (2016) Anaplastic hemangiopericytoma in the frontal horn of the lateral ventricle. Journal of Clinical Neuroscience 26:147-149.
- Tobias S, Kim CH, Kosmorsky G, Lee JH (2013) Management of surgical clinoidal meningiomas. Neurosurg Focus 14(6): e5.
- Lee JH, Jeun SS, Evans J, Kosmorsky G (2001) Surgical management of clinoidal meningiomas. Neurosurgery 48(5):1012-1021.
- Schiariti M, Goetz P, El-Maghraby H, Tailor J, Kitchen N (2011) Hemangiopericytoma: long-term outcome revisited. Clinical article. J Neurosurg 114(3): 747-755.
- Soni RS, Patel SK, Husain Q, Dahodwala MQ, Eloy JA, et al. (2014) From above or below: the controversy and historical evolution of tuberculum sellae meningioma resection from open to endoscopic skull base approaches. J Clin Neurosci 21(4): 559-568.
- Wilson DA, Duong H, Teo C, Kelly DF (2014) The supraorbital endoscopic approach for tumors. World Neurosurg 82(1-2): e243-256.
- Lucas JW, Zada G (2016) Endoscopic Endonasal and Keyhole Surgery for the Management of Skull Base Meningiomas. Neurosurg Clin N Am 27(2): 207-214.
- Daniel Walter Zumofen, Jonathan Rychen, Michel Roethlisberger, Ethan Taub, Daniel Kalbermatten, et al. (2017) A Review of the Literature on the Transciliary Supraorbital Keyhole Approach. World Neurosurg 98:614-624.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




