Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2690-5760
Research Article(ISSN: 2690-5760) 
Effeteness and Correctness of UDI Direct Marking on Steel Instruments During Surgical Trays Assembling Volume 2 - Issue 2
Mei Yun Yu*
- School of Nursing, Fooyin University, Taiwan
Received: June 04, 2020 Published: June 16, 2020
Corresponding author: Mei Yun Yu, School of Nursing, Fooyin University, Taiwan
DOI: 10.32474/JCCM.2020.02.000131
Abstract
The purpose of this project was to improve the efficiency and accuracy of surgical instrument packaging. The traditional
surgical tray assembly involves the technicians visually inspecting the appearance of each instrument. An experienced technician
can recognize most types instruments in this way, however junior technicians may need help to accomplish this.
The aim of direct marking on steel instruments is both quantity and instruments can be confirmed when the reader reads on
the marking. When using the traditional inspection process, staff may not recognize similar instruments with minor differences and
may place them into the wrong trays. An incorrect instrument in a tray may cause problems for the surgeon using the instruments.
Using Unique Device Identifiers allows the technicians to recognize the instruments by reading the markings to improve accuracy.
However, this process may make the process of assembling surgical trays take longer for the technician to complete.
Introduction
Surgical instruments are essential when surgeons perform
surgeries. Any successful surgery could not be performed
without fully functional instruments supply chain. The entire
functional supply chain is the hero behind the successful surgery’s
performance. Central supply rooms of hospitals manage the vast
quantity and various types of instruments to match the needs of
various surgeries. The management processes are complicated,
including checking over instruments with operation room nurses,
pre-cleaning, cleaning, and checking over before packaging,
sterilization, storage, and distribution. Malfunction of any stage
of management processes would raise the cost, delay or extend
surgeries, deduct the quality of service and harm for patients. The
entire fully functional supply chain is operated enough quantity of
instruments, capable human power and accelerated informatics
system to provide proper instruments for surgeries on time.
Steel instruments are the major portion of surgical instruments
[1]. Reprocessing steel surgical instruments is cruel for surgeries
preparation. The reprocessing steps include decontamination,
cleaning, assembling, sterilization, and storage.
Description of the problem
Supplying the correct and functional instruments for the
surgeon is the primary task of central supply room (CSR) staffs.
Most of the surgeons perform surgeries by their way, with their
instruments and material. The project implements in a medical
center in the north of Taiwan, CSR provides thirteen sub-specialists
of surgeons perform surgeries, 590 instrument trays/14320
instruments in total. Furthermore, some problem happens between
CSR and OR as follow:
a) The wrong number of instruments in surgical trays, doubt
of retained instrument issues raised.
b) The right instrument in a surgical tray, but it is not the one
surgeon in need.
c) Various types of instruments extend the training courses
of CSR staffs.
d) CSR staffs assemble the wrong instruments into trays
when the appearance of instruments is similar.
For that reason, simplifying the workflow and improving the correctness are important issues of the CSR.
The status is that surgical instruments trays connected to the bar-coding system in 2010. The function of the bar-coding system includes:
a) Categorized and connected to scheduling informatics
system to deliver correct case cart to the right operation room.
b) Quality management of sterilized instruments to track
proper method of sterilization and expired day automatically.
c) Keep the record of surgical trays to track the usage status
of every single tray.
Description of the setting of the project
The Ministry of Health and Welfare of Taiwan claimed “UDI
guideline of Taiwan” on 30th, OCT, 2015, to meet international
criteria and improve management efficiency of the medical device
in Taiwan. For patient safety and quality control reason, this
project includes direct marking on surgical instruments to improve
the correctness and effectiveness of pre-operative instrument
packaging procedure.
According to FDA regulation of medical devices, there are three
classifications of medical devices, and steel surgical instruments
belong to class I. Based on Federal Food Drug & Cosmetic (FD&C)
513(a) rule of United States: UDI direct marking on reusable devices
and instruments can be recognized between reprocessing.
The instruments mix and scatter in the tray when the automatic
cleaning machine works. If instruments of the tray mix with some
with the other tray, the packaging procedure will be longer and
more complicated.
Goals of the project
This project implemented UDI direct marking on steel
instruments, the purposes of the project are as follow:
a. Build up “instrument storage system.”
b. “Traceability of instruments used by patients with
contagious disease management.
c. Build up “record Instruments maintenance history.»
d. “Quality control of surgical trays assembling,” to improve
efficiency and accuracy.
e. “Employee educational material,” complied with photos of
instruments to raise up the competence of the employee.
The purpose of printing UDI direct marking on surgical
instruments is to explore the efficiency of assembling and
preparation. Both of efficiency and correctness are better than
inspection by staffs. The expectation of UDI direct marking integrates with informatics system to build up a learning platform
to reduce the learning course of the novice in the future. At the
meant time, the staff can correct the mistakes at the same time
when it is happening. By this way, it can reach two benefits of UDI
direct marking, reduce the stress of staff and improve healthcare
quality and patient safety.
Relevant guidelines and regulations: UDI regulation on healthcare industry
In July 2012, the FDA proposed the regulation: require all
medical devices to be labeled with a unique device identifier (UDI)
to allow tracking with automatic identification and data capture
(AIDC) technology. One year after the regulation proposed, all class
III medical devices (pacemakers, heart valves…) will be required to
be UDI-labeled, and class I devices, including surgical instruments,
will under the proposition three years later, 2015 [3]. The benefit
of UDI on surgical instruments is traceability on pre-cleaning,
cleaning, packaging, and distribution to operation room’s stages.
Direct marking, enabling the identification of every surgical
steel instrument, is expected to improve the reliability of the
processes. Furthermore, it improves the traceability would enhance
not only patient safety by preventing instruments retained in the
wound after surgery, but also cost efficiency by identifying correct
instruments during preparation and deducting the additional
instruments [1].
Based on the FDA regulations of reusable devices or
instruments requires readable UDI between uses, including the
UDI marking, shall be on the surface of the product and be readable
after each reprocessing. The PI (serial number) number should be
recognized by the manufacturer’s management system. The health
care providers shall meet these objectives and guidance to match
the regulation [2]. The UDI system can make the reorganization of
instruments more efficient and effective than traditional method”
the eyes of the human being.”
The material of UDI direct marking on steel surgical instruments
are described in UDI guidance of IMDRF (international medical
device regulation forum) and FDA UDI rules. The guideline of direct
marking on steel surgical instruments, such as kelly, mosquito,
knife handle, retractor, and scissors, meets the GS1 standard and
considerate repetitive reprocessing procedures. Nine sections of
the guideline should be followed:
a) Introduction
b) Conditions necessary for direct marking of twodimensional
symbols on steel instruments
c) Material suitable for marking and marking methods
d) Surface finishing and marking qualification for steel
e) Various marking and their adequacy
f) Marking quality
g) Attention for marking technique
h) Manufacturing responsibility and user responsibility
associated with marking
i) Companies that provide cooperation to prepare this
guideline and their devices
There are 18 of direct marking methods included in ISO/IEC
TR24720 (Information technology - Automatic identification and
data capture techniques Guidelines for direct part marking (DPM)).
Because of durability, laser method and the dot peen method were
recommended by the guideline. Direct marking on steel instruments
aimed to the extent the UDI regulation all over the world to improve
the quality of healthcare [2-4].
The expectation of direct marking on steel marking is
based on the fast-readable identifier on ordering efficiency and
standardization of assembling surgical instruments as a set. The
unique individual identifier recognizes the instruments more
efficient and corrective; it is possible to reconcile the surgical
instruments as a set by managing to computerize menu of the
surgical tray. In this way, UDI can significantly improve the accuracy
of surgical instruments tray. The correctness can improve the
patient safety during surgery by avoiding retaining foreign body
in the wound. Furthermore, any member can easily handle this
preparation, less expertise needed [5].
Project methods
According to FDA regulation of medical devices, there are three
classifications of medical devices, and steel surgical instruments
belong to class I. Based on Federal Food Drug & Cosmetic
(FD&C) 513(a) rule: UDI direct marking on reusable devices and
instruments can be recognized between reprocessing [6].
According to “Quality Management Checklist” of central supply
room (CSR) in November 2015 of a medical center locates in the
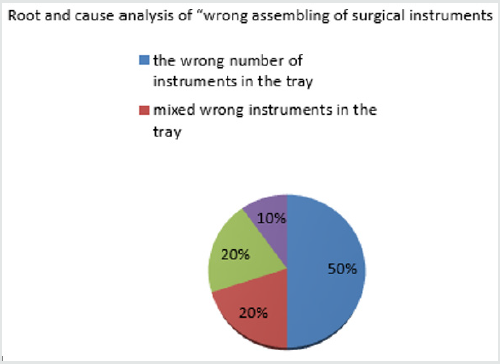
north of Taiwan, found ten mistakes of wrong assembling, 0.13%.
Five of them were “ the wrong number of instruments in the tray,”
two were “mixed wrong instruments in the tray,” two were “right
category but wrong size instrument” and one was “instrument
list didn’t update.” The average assembling time was 7 minutes
15 seconds, debugging of mixed instruments was 10 minutes
40 seconds. Random Quality Check of “mixed trays “proceeded
ten times per week, 20% mistakes could be debugged before
assembling (Figure 1).
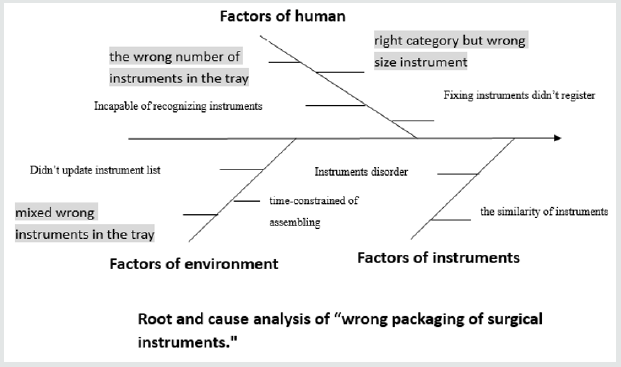
In general, the CSR provides 590 surgical trays, 14320 items in total and supplies 260 trays daily basis in average. The CSR staffs visually inspect the instruments before assembling. But the instruments mix and scatter in the tray when the automatic cleaning machine works. If instruments of the tray mix with some instruments of another tray, the assembling procedure will be longer and more complicated to inspect the correct instruments (Figure 2).
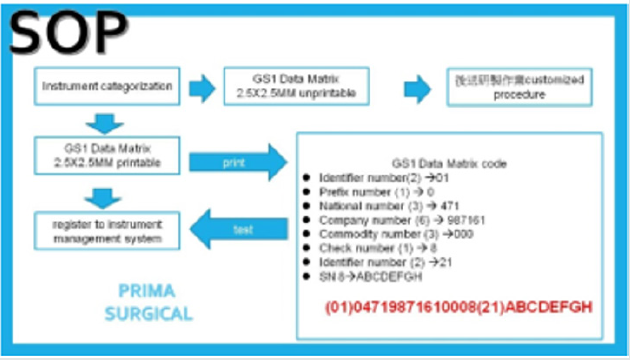
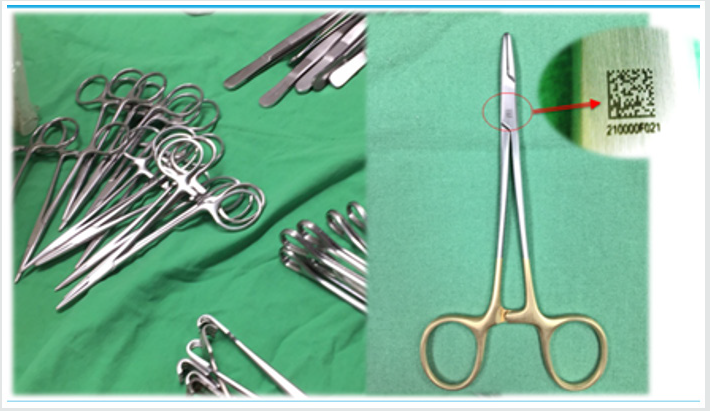
As a unique device identification (UDI) for metal instruments we have employed PRIMA Co. Ltd., laser printed 2.5mmx2.5mm square in size, two-dimensional Data Matrix on proper surface location the of the instruments, according to the guideline of GS1 (Figure 3).
The first step of standard operating procedures is «instrument categorization,» includes GS1 Data Matrix 2.5x2.5mm printable and GS1 Data Matrix 2.5x2.5mm unprintable. The second step of printable category instruments, laser prints GS1 Data Matrix 2.5x2.5mm on the surface of instruments. The Data Matrix includes thirty numbers in total-identifier number(2), prefix number(1), national number(3), company number(6), check number(3), identifier number(2) and serial number(6). The third step of printable category instruments, registration to instrument management system (Figure 4).
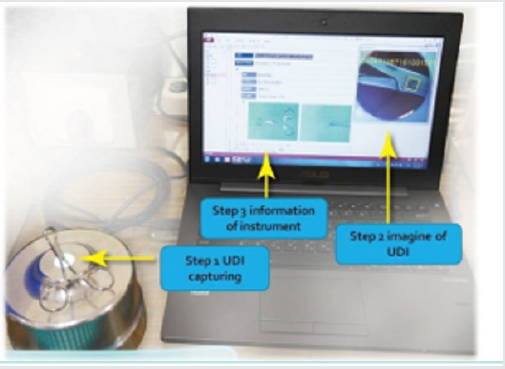
The second step of GS1 Data Matrix 2.5x2.5mm unprintable instruments follows the customized printing procedure. The third step is registration to instrument management system. After printed all UDI direct marking on the instrument, created traceable instrument UDI Data Matrix profile and registered to the management system. CSR staffs can capture the profile through the reader (Figure 5).
The Data matrix capturing includes three stages, capturing the UDI identifier by the reader, showing up the image of the instrument and proving the information of the instrument in the instrument management system [7].
Data Collection and Analysis Methods
The quality improvement project was designed by Head nurse and clinical instructor to improve the efficiency and correctness of assembling surgical instrument trays. We preceded ten testing trays per week during 1st July 2016 to 31st July 2016. There were 40 trays in total. There were 20 trays in 40 trays assembled by UDI identifier and the other 20 trays assembled by inspection of staffs. The efficiency comparison measures the time difference to assemble a surgical tray by staff’s inspection and UDI reader. The correctness test compares the percentage difference to find out a wrong instrument in the testing tray (Figure 6).
Results
This project compares the assembling instruments of 40 testing trays by UDI identifier and inspection by staffs. The average time of UDI identifier is 10 minutes 7 seconds and inspection of staffs is 7 minutes 1 second. The UDI identifier group is 3 minutes 6 seconds longer than the group of inspection of staffs. The average time of UDI identifier group is 144.18% of the group of inspection by staffs. The UDI identifier group needs an extra-step, reading, and this step takes a little more time than the group of inspection by staffs [8-10]. The average time of debugging mixed wrong instruments in testing trays of UDI identifier is 3 minutes 25 seconds, and inspection of staffs is 10 minutes 40 seconds. The UDI identifier group is 4 minutes 15 seconds shorter than the group of inspection of staffs. The average time of the group inspection by staffs is 312.20% of the group of UDI identifier. The average UDI identifier correction rate of a testing tray is 100%, and the average correction rate of inspection of staffs is 80%. The correctness rate of the UDI identifier group is 80% higher than the group inspection by staffs. In conclusion, the group of UDI identifier is more corrective than by the group inspection by staffs (Table 1).
Discussion
UDI direct marking on reusable instruments is a trend of the
central supply room, leading to FDA regulation. The effect of this
policy hasn’t found in the literature yet. Through this project, we
found UDI direct marking is high correctness rate at the moment
of reading because every single instrument has its’ own marking
and reader recognizes immediately. The reader is easy to detect the
difference of instruments. The other group, inspection by staffs, the
correctness rate is various, depends on the experience or personal
character of staff. But this project didn’t include the variety of staffs’
factors, so couldn’t appeal the real evidenced-based impaction. For
this reason, we can find the group of inspection of staffs takes a
longer time on” Debug mixed the wrong instrument to testing tray»
and achieves lower correctness rate on “Debugging correctness
rate of testing trays.»
The group of UDI identifier takes a longer time on “Assembling
instruments of the testing tray” than the group of inspection by
staffs, because of one more step, reading, and the central supply
room needs more workforce to assemble the surgical trays. Both
of laser printed direct marking on every steel instrument and
constructed the registered management system are the highly
financial expenditure of a hospital. In this project didn’t explore the
cost effectiveness of UDI direct marking on steel instruments. This
topic is worth to explore in the future [11].
Conclusion
Direct marking on reusable metal instruments is a new
issue of surgical instruments management. There is insufficient
literature discussion. Even the group of UDI direct marking takes
longer time during assembling the surgical testing tray, might
need more workforce working in a central supply room. But the
high correctness of assembling instruments is worthy. There are
possible benefits of UDI direct marking on reusable instruments
worth to discuss in the future.
The first benefit is “reduce employee training cost”, UDI direct
marking simplified the experience to recognize instruments and the
automatic identification would shorten the training course of staffs
to reach same, even higher correctness than current assembling
methods.
Another benefit is “holistic efficiency”: Even the automatic identification adds one stop more than prior assembling procedures, but the correctness will compensate the longer time spending procedures would improve overall efficiency. The benefit of releasing stress of employee: Through tracking, record to clarify the root of mistakes and reduce the stress from wrong packaging. Reduce the risk of medical malpractice
References
- Kyros Ipaktchi, Adam Kolnik, Michael Messina, Rodrigo Banegas (2013) Meryl Livermore and Connie Price. Current surgical instrument labeling techniques may increase the risk of unintentionally retained foreign objects: A hypothesis. Patient Safety in Surgery 7(31): 1-5.
- GS1 Healthcare Japan Surgical Instrument Marking Operations Guide GS1. Healthcare Japan Instrument Marking WG p. 1-71
- Chikayuki Ochiai(2009)RFID and barcode based management of surgical instruments in a theatre sterile supply unit. Healthcare Reference Book.p1-4
- S. Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health, Center for biologics evaluation and research (2015) Unique Device Identification: Direct Marking of Devices, Draft Guidance for Industry and 5Food and Drug Administration Staff. p.1-12.
- GS1 healthcare US (2014) U.S. FDA Unique Device Identification (UDI) Frequently Asked Questions (FAQS) R2 p. 1-23.
- IMRDF UDI working group (2013) International medical service regulators forum p1-19.
- Good Design Practice for Medical Devices and Equipment – Requirements Capture. Sandra Shefelbine, John Clarkson, Roy Farmer & Stephen Eason
- Med-Trace: Traceability Assessment Method for Medical Device Software development. Valentine Casey & Fergal Mc Caffery, Regulated Software Research Group Dundalk Institute of Technology & Lero - The Irish Software Engineering Research Centre.
- Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling, Guidance for Industry and Food and Drug Administration Staff (2015) U.S. Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health & Center for Biologics Evaluation and Research, USA.
- https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/UniqueDeviceIdentification/CompliancedatesforUDIRequirements/default.htm
- https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/UniqueDeviceIdentification/CompliancedatesforUDIRequirements/default.htm

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...