Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2690-5760
Review Article(ISSN: 2690-5760) 
Clinical and Laboratory Predictors for Total Antibody Against SARS-Cov-2 Among Convalescent Plasma Donors Volume 3 - Issue 3
Khin Phyu Pyar1*, Kyaw Myo Tun2, Kyaw Wunna3, Soe Win Hlaing4, Hein Htet OO5, Aung Aung6, Zarni Htet Aung7, Nyan Lin Maung8, Thurein Win9, Aung Phyoe Kyaw10, Kyaw Zay Ya11,Htet Aung12, Myo Thant Kyaw13 and Zay Phyo Aung14
- 1Professor and Head/Senior Consultant Physician, Department of Medicine/ Department of Nephrology, Defense Services Medical Academy, Defense Services General Hospital, Myanmar
- 2Associate Professor, Preventive and Social Medicine Department, Defense Services Medical Academy, Myanmar
- 3Consultant Microbiologist, Defense Services General Hospital, Myanmar
- 4Senior Consultant Physician, Department of Medicine, Defense Services General Hospital, Myanmar
- 5Consultant Hematologist, Military Hospital, Myanmar
- 6Consultant Hematologist, Defense Services General Hospital, Myanmar
- 7Consultant Physician, Department of Medicine, Military Hospital, Myanmar
- 8Consultant Physician, Department of Medicine, Military Hospital, Myanmar
- 9Assistant Lecturer / Oncologist, Department of Medicine, Defense Services Medical Academy, Myanmar
- 10Assistant Lecturer /Consultant Physician, Department of Medicine, Defense Services Medical Academy, Myanmar
- 11Assistant Lecturer/Hematologist, Department of Medicine, Defense Services Medical Academy, Myanmar
- 12Department of Pathology, Defense Services General Hospital, Myanmar
- 13Assistant Lecturer, Department of Medicine, Defense Services Medical Academy, Myanmar
- 14Assistant Lecturer, Department of Medicine, Defense Services Medical Academy, Myanmar
Received:July 21, 2021 Published: August 05, 2021
Corresponding author:Khin Phyu Pyar, Professor and Head/Senior Consultant Physician, Department of Medicine/ Department of Nephrology, Defense Services Medical Academy, Defense Services General Hospital, Myanmar
DOI: 10.32474/JCCM.2021.03.000164
Abstract
Background: We studied clinical, laboratory parameters and total antibody against SARS-CoV-2 among COVID-19 convalescent plasma donors.
Methods: We analyzed 302 serum specimens from 1335 candidates recovered from confirmed COVID-19 infection. Their clinical characteristics: symptoms, age, sex, body mass index, blood group, blood pressure, glycaemic status and renal function were related with SARS-CoV-2 total antibody level; it was measured twice with 4 weeks apart with the use of E411 Fully Automated Immuno Analyzer.
Findings: There was no relation between SARS-CoV-2 total antibody level with symptoms, age, sex and blood group, high blood pressure and diabetes mellitus. The percentage distribution of high total antibody level was significantly higher in candidates with blood group “Non-O” group (77.9%) than blood group “O” group (22.1%). (p= 0.03) Adequate total antibody level was detected in 22.9% in underweight group, 28.7% in normal BMI group, 45.5% in overweight group and 50% in obese group. There was positive relationship between BMI and adequate total antibody level (p = <0.001). Mean antibody level of participants with raised serum creatinine was 16.82 ± 15.28 COI; it was nearly half of those with normal renal function (33.58 ± 32.6 COI). Antibody levels measured at 70 days (mean=87.27 ± 25.96 COI) was significantly higher than that of 42 days (mean= 56.19 ± 20.23 COI).
Conclusion: The best predictor for adequate total antibody against SARS-CoV-2 were those candidates with normal or high BMI status, “non-O” blood group and normal serum creatinine; the plasma should be collected at 70 days symptom onset after COVID-19 infection.
Introduction
Plasma from people recovering from infection: bacteria, viral or protozoa, may have pathogen specific antibodies which may fight to the same pathogen if they are given to patients with same disease. This concept has been applied since 1890; West Nile virus [1], Ebola virus [2] Spanish flu, influenza, measles, pertussis and diptheria, especially before the adventure of vaccine or antimicrobial therapy [3]. No one cannot deny the fact that many lives were saved with the help of plasma especially in pandemic diseases. However, the exact timing after infection for the maximum antibody level was described vaguely as 6 weeks to 10 weeks. Moreover, the clinical predictors to get high antibody level were mentioned in different report with some controversies. In COVID-19 pandemic, plasma from convalescent patients was tried as one of therapeutic options; US Food and Drugs Administration authorized its emergency use for hospital patients with covid-19 in August 2020 [4,5]. Antibodies against SARS-CoV-2 are produced naturally from subjects who acquired COVID-19 infection and the level is maximum at 4-6 weeks after infection [6,7]. These antibodies will promote recovery in patients with severe COVID-19 infection. Measuring antibody level in all cases with COVID-19 infection is expensive; it is not easy especially in developing country. Moreover, the relationship between clinical characteristics and antibody response to COVID-19 is not clearly defined and some issues are controversy. Therefore, finding the relation between clinical characteristics: symptoms, age, sex, body mass index, hypertension, diabetes mellitus, blood group and serum creatinine, and the antibody level against SARS-CoV-2 among COVID-19 convalescent plasma donor candidates would be useful for selection of donor for convalescent plasma in the future. Moreover, the timing for collection of plasma to get the best total Ab level; 42 days or 70 days was also important for therapeutic aspect of convalescent plasma therapy.
Research Procedure
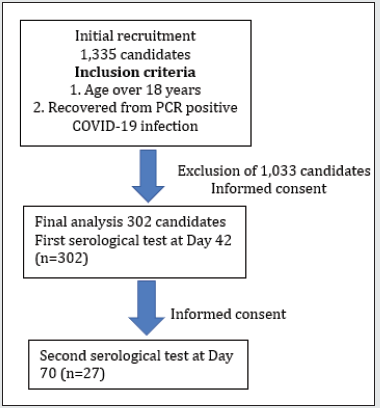
This study aimed to find out the relation between clinical characteristics: age, sex, body mass index, hypertension, diabetes mellitus, anaemia, blood group, serum creatinine, and the antibody level against SARS-CoV-2 among COVID-19 convalescent plasma donor candidates; therefore, we could choose the best clinical and laboratory predictors. We recruited COVID-19 confirmed patients from various military COVID treatment centers and quarantine centers: Defence Services Liver Hospital, Indaing Hospital & Navy hospital, Ba Yint Naung, Kyauk Taw Gyi and Defence Services Medical School quarantine center; and we made contact with phone or viber to get informed consent for convalescent plasma donation and this study. After getting informed consent verbally, an appointment was made for clinical and blood examination. History taking particularly clinical features of COVID-19 infection; fever, cough, dyspnea, changes in smell, changes in taste, abdominal pain and diarrhea was done at 6 weeks after symptom onset of COVID-19 infection. Physical examination was performed for anaemia, blood pressure and general condition. Then, 5 cc of venous blood was taken for total antibody level, random blood sugar, serum creatinine and blood group. Clinical characteristics like age, sex, body weight, height, immune status: diabetes mellitus, on steroids, on immunosuppressants and transplant recipients, and hypertension, malignancy, renal function and blood group were recorded in the proforma. SARS-CoV-2 total antibody was measured with the use of E411 Fully Automated Immuno Analyzer [8]. Adequate total Ab or high total Ab titer was defined if the total Ab level was ≥1:40 COI. A total of 302 participants were studied at 42 days and the blood sample from those willing to give informed consent for second examination were taken again at 70 days since symptom onset. Ethical approval was obtained from “Hospital Ethics and Research Committee of No (1) Defence Services General Hospital (1/1000) Mingaladon, Myanmar”. Data was analysed by using SPSS software version (23).
Results
Although 1335 candidates were contacted for informed consent for donation of plasma and participation in this study, 1033 were excluded as they had malignancy, tuberculosis, renal transplant recipient, aplastic anaemia, leukaemia and autoimmune disease- SLE. Baseline clinical characteristics including age, sex, blood group, BMI, serum creatinine, SARS CoV2 total antibody status and presence of COVID-19 symptoms, shown in Table 1 and the relation between clinical characteristics and antibody levels were studied, shown in Table 2. Half of the candidates were symptomatic and only 30% of them had significant total Ab level (total Ab titers more than 1:40). Mean total Ab level of the candidates was 32.64 COI (range 0.06 COI- 162.10 COI). Twenty five percent of candidates had high Ab titers ≥1:40 COI. And half of the candidates having total Ab titers more than 1:40 COI were symptomatic. There was no relation between severity of symptom and significant total Ab level. The mean age was 30 years, and the range was 18 to 78 years. Majority of the candidates (80.8%) were younger age group (18- 40 year) and (41-60 year) age group was 19.2%. Although mean total Ab level of both age groups were more or less the same and 80% of participants whose total Ab level > 40 COI were younger age group (18-40 year), it was not statistically significant. Eighty three percent of the candidates were male; there was no relation between sex and total Ab level. Regarding blood group, the proportion of blood group of the candidates was highest in blood group “O” (30.5%), followed by blood group “B” (30.1%) and blood group “A” (28.8%). The lowest contribution was blood group “AB” (10.6%). It was in accordance with blood group distribution pattern of general population in Myanmar. There was no relation between blood group and significant Ab level. Nevertheless, the difference in total Ab level was interesting if we categorized the four blood groups, “A”, “B”, “AB” and “O”, into two main groups: blood group “non-O” (n=210) and blood group “O” (n=92) group. The mean total Ab level of candidates with blood group “non-O” group was higher than that of “O” group: 33.6 COI ± 32.7 COI and 30.4 COI ± 30.5 COI respectively.
Table 1: Baseline clinical characteristics of covid-19 Convalescent plasma donor candidates (n=302).
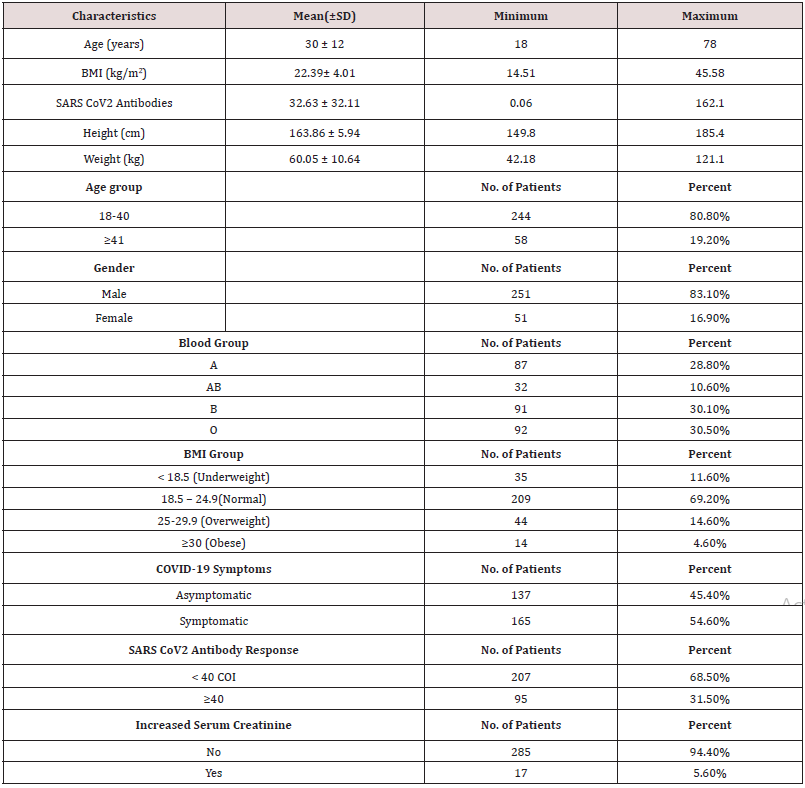
Table 2: Association between clinical characteristics and SARS CoV2 Antibody responses among COVID-19 convalescent plasma donor candidates (n=302).
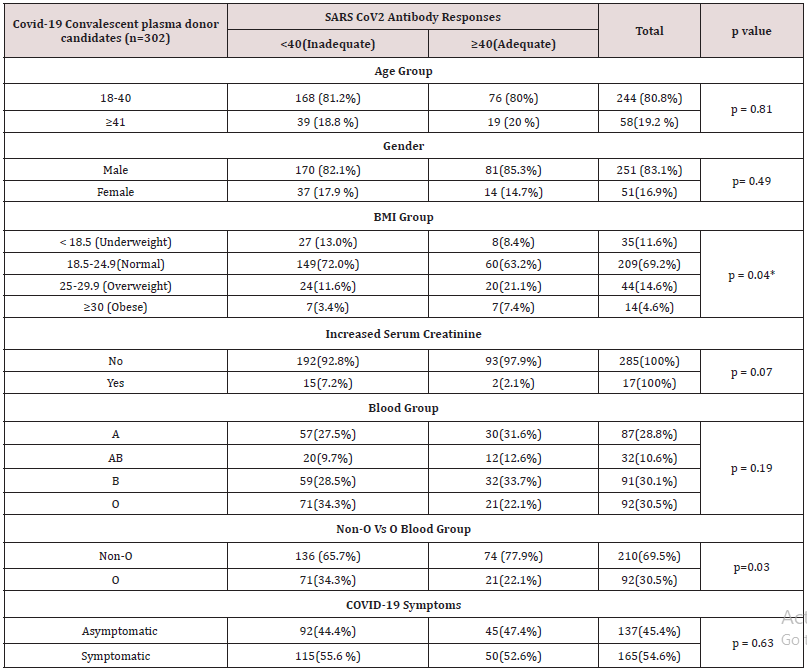
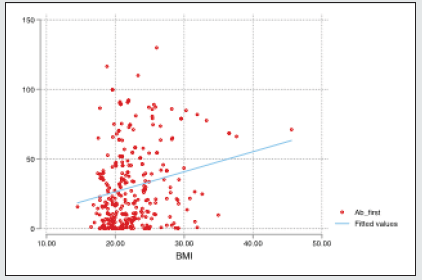
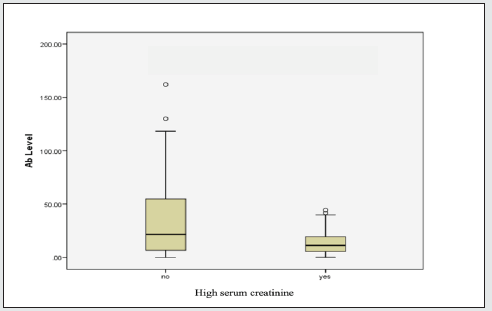
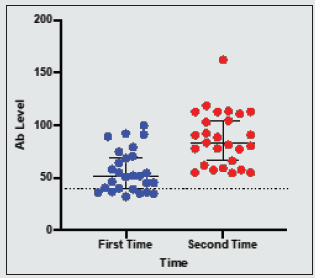
Moreover, the percentage distribution of high total Ab level was significantly higher in candidates with blood group “non-O” group (77.9%) than blood group “O” group (22.1%). (p= 0.03). Mean BMI of the candidates was 22.4 (range 14.5- 45.6). The significant total Ab level was the highest (63.2%, 60/95) in those with normal BMI (18.5-24.9). The proportion of significant total Ab level in overweight group (BMI 25-29.9) and underweight (BMI less than 18.5) group was 21.1% (20/95) and 8.4% (8/95) respectively. In obese group (BMI over 30), only 7.4% (7/95) had good total Ab response (>1:40 COI) (p= 0.04). Regarding the relationship between total Ab level and BMI, significant total Ab level was detected in 22.9% (8/35) in underweight (BMI less than 18.5) group, 28.7% (60/209) in normal BMI (18.5-24.9), 45.5% (20/44) in overweight group (BMI 25-29.9) and 50% (7/14) in obese group (BMI over 30). Figure 1 revealed that there was positive relationship between BMI and significant Ab level. (p=0.0003). There was no relation between other metabolic state (diabetes mellitus, hypertension) and total Ab response. In-view of renal function, among the candidates, 5.6 % (17/302) of candidates had raised serum creatinine. Only 11.8% (2/17) of candidates had significant total Ab level. Mean total Ab level of the candidates with normal serum creatinine was 33.58 COI and mean total Ab level of the participants with raised serum creatinine was half of those participants with normal renal function (16.82 COI). There was a negative correlation between total Ab level and serum creatinine (Figure 2). Those with high serum creatinine had negative correlation with SARS-CoV-2–specific total antibody and we should screen serum creatinine for recruiting convalescent plasma therapy. The total antibody level measured at 70 days (mean= 87.27 COI ± 25.96 COI) was significantly higher than that of 42 days (mean= 56.19 COI ± 20.23 COI). The difference was statistically significant (p=<0.001) (Figure 3).
Discussion
SARS-CoV-2–specific antibody titer may correlate with COVID-19 disease severity, age, sex, BMI, hypertension, anaemia, diabetes mellitus, blood group and serum creatinine. It helps viral clearance early, reduces the severity and shortens hospital stay; thus, it is the rationale for administration of plasma as a treatment for COVID-19. There are controversies in clinical predictors for selection of plasma donor and its durability. Although the studies done in Germany [9,10] and US had agreement on the relation between SARS-CoV-2–specific antibody titers and some clinical characteristics, there were some disagreements [11]. According to [7], a history of higher number of COVID-19 symptoms and higher levels of anti-SARS-CoV-2 IgG and IgA antibodies in immunoassays can preselect donations with higher neutralizing capacity; other clinical characteristics like age, sex, BMI and blood group type did not predict Ab level. Moreover, Masiá et al. (2021) mentioned that SARS-CoV-2 viral load predicted the Ab response. The reports on the relation between Ab with BMI, blood group [12] and serum creatinine was rare. We wondered factors predicting the response - SARS-CoV-2–specific total antibody in Myanmar population with previous COVID-19 infection. Male sex over 50 year were found to have severe infection in several studies [13-15] and their Ab level were expected to be high too. Old age, male sex and clinical severity correlated well with high Ab titers in some report from US and Germany; the finding by study from Spain overlooked it. In this study, half of the participants were symptomatic and 52.6% of candidates with symptoms had adequate Ab level for convalescent plasma donation (Ab level more than 40 COI). There was no relation between symptom and adequate Ab level. Thus, our findings fail to note the report from US [10] and Germany [9] where adequate Ab level was related with severity of symptoms. However, showing no relation between Ab response in this study confirm the finding by [11].
The mean age in this study was 30 year (range 18-78 year); the majority (80.8%) were younger age group (18-40 year). Although 80% of participants whose Ab level > 40 COI were younger age group (18-40 year), it was not statistically significant. This finding also disregards others [9,10] older age group in their study population had relatively high Ab level. Eighty three percent of the subjects were male. There was no relation between sex and Ab level; this finding proves that of Spanish study [11]. It again overlooks the finding by [9,10]. Relation between the type of blood group and its susceptibility as well as severity of SARS-CoV-2 infection was interesting. Most of the finding concluded that those with blood group O had protection against SARS-CoV-2 [16-18]. In view of severity, [17] pointed out that those with blood group A were more prone to have severe infection; however, Almadhi et al neglected it- no association was observed between blood group and the risk of a severe ICU-requiring infection. According to [17], those with blood group A probably have high Ab level because candidates with severe infection were likely to have good Ab response. Shokri et al demonstrated that non-O blood group carriers with COVID-19 were at higher risk of developing severe outcomes in comparison to O blood group; therefore, non-O blood group carriers were expected to have high Ab level. Regarding blood group, the proportion of blood group of the candidates was highest in blood group “O” (30.5%), followed by blood group “B” (30.1%) and blood group “A” (28.8%). The lowest contribution was blood group “AB” (10.6%). It was in accordance with blood group distribution pattern of general population in Myanmar. There was no relation between blood group and significant Ab level. Nevertheless, the difference in total Ab level was interesting if we categorized the four blood groups, “A”, “B”, “AB” and “O”, into two main groups; blood group “Non-O” (n=210) and blood group “O” (n=92) group. The mean total Ab level of candidates with blood group “Non-O” group was higher than that of “O” group: 33.6 COI ± 32.7 COI and 30.4 COI ± 30.5 COI respectively. Moreover, the percentage distribution of high total Ab level was significantly higher in candidates with blood group “Non-O” group (77.9%) than blood group “O” group (22.1%). (p= 0.03) Therefore, in this study, not only the mean Ab level but also the proportion of adequate Ab response of candidates with blood group “Non-O” was higher than that of “O” group; this finding agree the report by [12] where those with blood group “AB” had higher Ab level than those with blood group “O”. It also provides evidence for relation between clinical severity and blood group finding by [16,17]; blood group “Non-O” were more likely to get severe SARSCoV- 2 infection and higher Ab level. Interestingly, those with BMI over 30, obesity, were found to have severe SARS-CoV-2 infection and high mortality in previous studies [19-22] and it was more susceptible to infection and found to have more viral load [23]. Nevertheless, they had higher Ab level than non-obese [24]. The good point was that they became excellent candidates for plasma. In this study, mean BMI was 22.4 kg/m2 (range 14.5- 45.6). The adequate Ab level was highest (63.2%, 60/95) in those with normal BMI (18.5-24.9). The adequate Ab level of overweight group (BMI 25-29.9) and underweight (BMI less than 18.5) group was 21.1% (20/95) and 8.4% (8/95) respectively. In obese group (BMI over 30), only 7.4% (7/95) had adequate Ab response (> 40 COI) (p= 0.04) (Table 2). Regarding relationship between Ab level and BMI, adequate Ab level was detected in 22.9% (8/35) in underweight (BMI less than 18.5) group, 28.7% (60/209) in normal BMI (18.5- 24.9), 45.5% (20/44) in overweight group (BMI 25-29.9) and 50% (7/14) in obese group (BMI over 30). There was positive relationship between BMI and adequate Ab level (p = <0.001).
There was no relation between metabolic state (diabetes mellitus, hypertension) and Ab response [25-27]. Many reports concluded that development of acute kidney injury in severe SARSCoV- 2 infection was one of the poor prognostic indicators [28]. The kidney function would have recovered by 42 days symptom onset. In this study, among the participants, 5.6 % (17/302) had raised serum creatinine; only 11.8% (2/17) of those with raised serum creatinine had adequate Ab level. Mean Ab level of participants with normal serum creatinine was 33.58 ± 32.6 COI and those with raised serum creatinine was 16.82 ± 15.28 COI which was nearly half of those with normal renal function. Thus, raised serum creatinine level may interfere with SARS CoV2 antibody level but it was not statistically significant. Therefore, we should screen serum creatinine for recruiting convalescent plasma therapy. Total antibody levels measured at 70 days (mean= 87.27 ± 25.96 COI) were significantly higher than that of 42 days (mean= 56.19 ± 20.23 COI). The difference was statistically significant (p=<0.001).
Conclusion
The best predictor for adequate total antibody against SARSCoV- 2 were those candidates with normal or high BMI status, “Non-O” blood group and normal serum creatinine; the plasma should be collected at 70 days symptom onset after COVID-19 infection.
Acknowledgements
The authors would like to thank all the candidates for giving informed consent to this study. The authors also acknowledged Prof Ko Ko Lwin, Prof Kyaw Zay Ya, Prof Myint Zaw for administrative support and Professor Tin Moe Mya for laboratory support.
Declaration of Conflict of Interest
The authors declared no potential conflicts of interests with respect to authorship and publication of this article.
Ethical Approval
Hospital Research and Ethics Committee of Defence Services General Hospital.
Funding
The authors received no financial support for publication of this article.
References
- Planitzer C B, Modrof J, Yu M W, Kreil T R (2009) West Nile virus infection in plasma of blood and plasma donors, United States. Emerging Infectious Diseases 15(10): 1668-1670.
- Van Griensven J, Edwards T, De Lamballerie X, Semple M G, Ebola Tx Consortium (2016) Evaluation of Convalescent Plasma for Ebola Virus Disease in Guinea. The New England Journal of Medicine 374(1): 33-42.
- Piero M, Cozza A, Silvestro G (2020) The true historical origin of convalescent plasma therapy. Transfus Apher Sci 59(5): 102847.
- Hu X, Hu C, Jiang D, Zuo Q, Li Y, et al (2020) Effectiveness of Convalescent Plasma Therapy for COVID-19 Patients in Hunan, China. Dose Response 18(4): 1559325820979921.
- Duan K, Liu B, Li C, Zhang H, Yu T, et al (2020) The feasibility of convalescent plasma therapy in severe COVID- 19 patients: A pilot study. MedRxiv.
- Li L, Tong X, Chen H, He R, Lv Q, et al (2020) Characteristics and serological patterns of COVID-19 convalescent plasma donors: Optimal donors and timing of donation. Transfusion 60(8): 1765-1772.
- Körper S, Jahrsdörfer B, Corman V M, Pilch J, Wuchter P, et al (2021) Donors for SARS-CoV-2 Convalescent Plasma for a Controlled Clinical Trial: Donor Characteristics, Content and Time Course of SARS-CoV-2 Neutralizing Antibodies. Transfus Med Hemother 48(3): 137-147.
- Chan C W, Parker K, Tesic V, Baldwin A, Tang N Y, et al (2020) Analytical and Clinical Evaluation of the Automated Elecsys Anti-SARS-CoV-2 Antibody Assay on the Roche cobas e602 Analyzer. American Journal of Clinical Pathology 154(5): 620-626.
- Schlickeiser S, Schwarz T, Steiner S, Wittke K, Al Besher N (2020) Disease Severity, Fever, Age, and Sex Correlate With SARS-CoV-2 Neutralizing Antibody Responses. Frontiers in Immunology 11: 628971.
- Boonyaratanakornkit J, Morishima C, Selke S, Zamora D, McGuffin S, et al (2021) Clinical, laboratory, and temporal predictors of neutralizing antibodies against SARS-CoV-2 among COVID-19 convalescent plasma donor candidates. The Journal of Clinical Investigation 131(3): e144930.
- Markewitz R, Torge A, Wandinger K P, Pauli D, Franke A, et al (2021) Clinical correlates of anti-SARS-CoV-2 antibody profiles in Spanish COVID-19 patients from a high incidence region. Scientific Reports 11(1): 4363.
- Madariaga M L L, Guthmiller J J, Schrantz S, Jansen M O, Christensen C, (2021) Clinical predictors of donor antibody titre and correlation with recipient antibody response in a COVID-19 convalescent plasma clinical trial. Journal of Internal Medicine 289(4): 559-573.
- Biswas M, Rahaman S, Biswas T K, Haque Z, Ibrahim B (2021) Association of Sex, Age, and Comorbidities with Mortality in COVID-19 Patients: A Systematic Review and Meta-Analysis. Intervirology, 64(1): 36-47.
- Li X, Marmar T, Xu Q, Tu J, Yin Y, et al (2020) Predictive indicators of severe COVID-19 independent of comorbidities and advanced age: A nested case−control study. Epidemiol Infect 148: e255.
- Peckham H, Gruijter N M, Raine C, Radziszewska A, Ciurtin C, et al (2020) Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nature Communications 11(1): 6317.
- Shokri P, Golmohammadi S, Noori M, Nejadghaderi S A, Carson Chahhoud K, et al (2021) The relationship between blood groups and risk of infection with SARS-CoV-2 or development of severe outcomes: A review. Reviews in Medical Virology pp. 1-12.
- Izak M, Yahalom V, Maor Y, Zimhoni O, Cohen D, et al (2021) [COVID-19—BLOOD GROUPS, ANTIBODIES AND CONVALESCENT PLASMA]. Harefuah 160(3): 139-143.
- Yanardag Acik D, Bankir M (2021) Relationship of SARS-CoV-2 Pandemic with Blood Groups. Transfusion Medicine and Hemotherapy 48(3): 161-167.
- Tamara A, Tahapary D L (2020) Obesity as a predictor for a poor prognosis of COVID-19: A systematic review. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 14(4): 655-659.
- Drucker D J (2021) Diabetes, obesity, metabolism, and SARS-CoV-2 infection: The end of the beginning. Cell Metabolism 33(3): 479-498.
- Deng M, Qi Y, Deng L, Wang H, Xu Y, et al (2020) Obesity as a Potential Predictor of Disease Severity in Young COVID-19 Patients: A Retrospective Study. Obesity 28(10): 1815-1825.
- Chu Y, Yang J, Shi J, Zhang P, Wang X (2020) Obesity is associated with increased severity of disease in COVID-19 pneumonia: A systematic review and meta-analysis. European Journal of Medical Research 25(1): 64-69.
- Zakka K, Chidambaram S, Mansour S, Kamal Mahawar, Purkayastha S, et al (2021) SARS-CoV-2 and Obesity: “CoVesity”-A Pandemic Within a Pandemic. Obesity Surgery 31(4): 1745-1754.
- Soffer S, Glicksberg B S, Zimlichman E, Efros O, Levin M A, et al (2021) The Association Between Obesity and Peak Antibody Titer Response in COVID-19 Infection. Obesity doi:10.1002/oby.23208.
- Gallo Marin B, Aghagoli G, Lavine K, Yang L, Siff E J, et al (2021) Predictors of COVID-19 severity: A literature review. Reviews in Medical Virology 31(1): 1-10.
- Cekerevac I, Turnic TN, Draginic N, Andjic M, Zivkovic V, et al (2021) Predicting Severity and Intrahospital Mortality in COVID-19: The Place and Role of Oxidative Stress. Oxidative Medicine and Cellular Longevity 2021: 6615787.
- Zhang T, Huang W S, Guan W, Hong Z, Gao J, et al (2020) Risk factors and predictors associated with the severity of COVID-19 in China: A systematic review, meta-analysis, and meta-regression. J Thorac Dis 12(12): 7429-7441.
- Bhargava A, Fukushima E A, Levine M, Zhao W, Tanveer F, et al (2020) Predictors for Severe COVID-19 Infection. Clinical Infectious Diseases 71(8): 1962-1968.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...