Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Research Article(ISSN: 2637-4609) 
Phytochemical Screening and Proximate Analysis of Garlic (Allium Sativum) Volume 4 - Issue 1
Muhammad Ali1* and Idris S Ibrahim2
- 1Department of Microbiology, Federal University Gusau, Nigeria
- 2Department of Pharmaceutical Technology, School of Technology, Nigeria
Received: March 27, 2019; Published: April 29, 2019
*Corresponding author: Muhammad Ali, Department of Microbiology, Federal University Gusau, Nigeria
DOI: 10.32474/AOICS.2019.04.000180
Abstract
Natural products have been an integral part of ancient traditional medicine systems. The objective of the study was to investigate the phytochemical constituents and proximate composition of Garlic (A. sativum) extracts. The phytochemical screening of the Garlic for various phytochemical constituents was conducted using laboratory method. The proximate and mineral composition was determined using standard method. The qualitative phytochemical screening of Allium sativum aqueous and ethanol extracts indicated the presence of Alkaloid, terpenoids, flavonoids, steroid, phenol, Anthraquinones, saponin, tannin and glycoside. Quantitatively, Alkaloid was found to be the abundant constituent making about 7.2%, followed by Tannin and saponin constituting 4.8% and 4.3% respectively. The qualitative proximate composition of the bulb extract of Allium sativum bulb in g/100g showed the extract contain carbohydrate, protein, fats, fibre, moisture and ash while the quantitative analysis result was presented as carbohydrate with 66.00%, protein 16.23%, fats 2.44%, crude fibre 03.96%, moisture content 5.52% and ash content 05.85%. The mineral composition analysis of the bulb indicates the presence of calcium (23.40%), potassium (10.95%), magnesium (3.90%), zinc (0.44%), phosphorous (9.85%), iron (5.20%) and copper (0.05%). The presence of nutrients proves why A. sativum bulb can be used as food supplement.
Keywords: Antimony; Consciousness Energy Healing Treatment; The Trivedi Effect®; PXRD; Particle size
Introduction
Natural products have been an integral part of ancient traditional medicine systems, e.g. Chinese, Ayurvedic and Egyptian [1]. Over the years, they have assumed a very central stage in modern civilization as natural source of chemotherapy as well as amongst scientist in search for alternative sources of drugs. According to the World Health Organization [2], a medicinal plant is any plant which in one or more of its organs, contains substances that can be used for therapeutic purposes, or which are precursors for chemo-pharmaceutical semi synthesis. Such a plant will have its parts including leaves, roots, rhizomes, stems, barks, flowers, fruits, grains or seeds, employed in the control or treatment of a disease condition and therefore contains chemical components that are medically active [3]. Phytochemicals are bio-active chemicals of plant origin. They are regarded as secondary metabolites because the plant that manufactures them may have little need for them. They are naturally synthesized in all parts of the plant body; bark, leaves stem, root, flower, fruits, seeds, etc. i.e. any part of the plant body may contain active components [4].
Garlic (Allium sativum L.) is one of those plants that were seriously investigated over several years and used for centuries to fight infectious diseases [5]. It is belong to family Amaryllidaceae [6]. It is a cultivated food highly regarded throughout the world. Garlic is originally from Central Asia, and as one of the earliest of cultivated plants [7]. Therapeutic use of garlic has been recognized as a potential medicinal value for thousands of years to different microorganisms. For example; antifungal, antiviral, antibacterial antihelmantic, antiseptic and anti-inflammatory properties of garlic are well documented. Moreover, garlic extracts exhibited activity against both gram negative (E. coli, Salmonella sp. and Citrobacter Enterobacter, Pseudomonas Klebsiella) and gram positive (S. aureus, S. pneumonia, streptococcus and Bacillus anthrax) all of which are cause of morbidity worldwide [8].
In Africa, particularly in Nigeria, it is used to treat abdominal discomfort, diarrhea, otitis media and respiratory tract infections [9]. In Europe and India, it was used to treat common colds, hay fever and asthma [10]. In addition to its reputation as a healthy food, garlic has shown anti-viral, anti-bacterial, antifungal and antioxidant capacities. Additionally, anti-atherosclerotic and anti-cancer properties have also been demonstrated. Many researches had demonstrated its effectiveness and broad spectrum antimicrobial activity against many species of bacteria, viruses, parasites, protozoan and fungi [9]. Garlic extract inhibits the growth of Gram positive and Gram negative bacteria, such as Staphylococcus, Streptococcus, Micrococcus, Enterobacter, Escherichia, Klebsiella, Lactobacillus, Pseudomonas, Shigella, Salmonella, Proteus, and Helicobacter pylori [11]. The objective of the present study was to investigate the phytochemical constituents and proximate composition of Garlic (A. sativum) extracts.
Materials and Methods
Sample collection and identification of Garlic bulb
Fresh bulbs of Garlic Allium sativa (Family Amaryllidaceae) were purchased from Kano main Market in Kano city, Nigeria. Identification and authentication of the Garlic was done at Herbarium in the Department of Plant Science, Bayero University Kano with the following voucher number BUKHAN 297. Voucher specimens were deposited in the Herbarium for reference.
Preparation of extracts
The collected bulbs were washed with distilled water, air dried for two weeks and grounded into fine powder using sterile pestle and mortar under laboratory condition. Fifty (50) grams of the powder was mixed with 500ml of Distilled water and ethanol in a sterile conical flask separately and stand for 3 days with intermittent shaking. The mixtures were filtered using filter paper and concentrated in water bath at 70 °C for 3 hours. Each extract was kept in a sterile container and refrigerated at 4 °C for further experiment.
Phytochemical screening
The phytochemical screening of the Garlic for various phytochemical constituents such as terpenoids, flavonoids, alkaloids, reducing sugars, steroid, glycoside, phenol, Anthraquinones, saponin and tannin was conducted using standard methods as described by Sofowora [12] and Trease and Evans [13].
Quantitative phytochemical analysis
Different methods were used in evaluating the quantity of phytochemical constituents of the plant materials used. Spectrophotometric method was used to determine Terpenoids, tannins, steroids, anthraquinone, and glycosides. Folin-Ciocalteu procedure was used to determine phenol content. Flavonoids, alkaloids and saponins were determined by the methods described by Adeniyi et al. [14].
Proximate analysis
Proximate analysis of the Moringa leaves was conducted to determine the ash content; crude protein, crude fibre, crude lipid, carbohydrate and dry matter using methods described by Udo and Oguwele [15]; James [16] and Association of Official Analytical Chemist (AOAC) [17]. The proximate parameters were expressed in percentage (%).
Mineral analysis
The mineral composition of the leaves including potassium (K), calcium (Ca), magnesium (Mg), and zinc (Zn), phosphorous (P) and iron (Fe) were determined using the atomic absorption spectrophotometer, as described the methods of AOAC [17]. Phosphorus was determined colorimetry method.
Results
Phytochemical screening
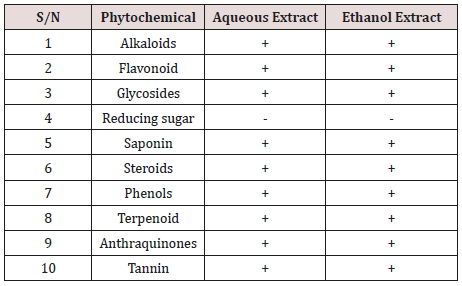
The qualitative phytochemical screening of Allium sativum aqueous and ethanol extracts is presented in Table 1. The result indicated the presence of Alkaloid, terpenoids, flavonoids, steroid, phenol, Anthraquinones, saponin, tannin and glycoside.
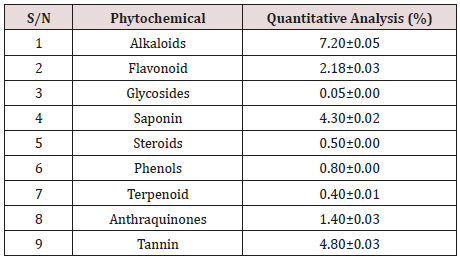
The qualitative phytochemical screening of Allium sativum extracts is presented in table below (Table 2). Quantitatively, Alkaloid was found to be the abundant constituent making about 7.2 %, followed by Tannin and saponin constituting 4.8 % and 4.3 % respectively.
Proximate analysis
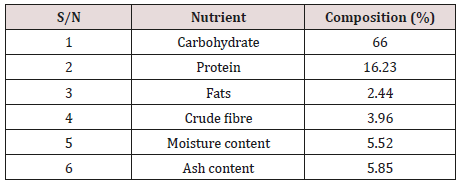
The qualitative and quantitative proximate analysis of Allium sativum bulb is presented in the table below (Table 3). The qualitative proximate composition of the bulb extract of Allium sativum bulb in g/100g showed the extract contain carbohydrate, protein, fats, fibre, moisture and ash while the quantitative analysis result was presented as carbohydrate with 66.00%, protein 16.23%, fats 2.44%, crude fibre 03.96%, moisture content 5.52% and ash content 05.85%.
Mineral analysis
The mineral analysis of Allium sativum bulb is presented in Table 4. The mineral composition analysis of the bulb indicates the presence of calcium (23.40%), potassium (10.95%), magnesium (3.90%), zinc (0.44%), phosphorous (9.85%), iron (5.20%) and copper (0.05%).
Discussion
The results of the present study suggested that several phytochemicals are present in Allium sativum bulb extracts. Phytochemicals give plants their colour, flavour, smell and are part of a plant`s natural defense system and protect them against herbivorous insects and vertebrates, fungi, pathogens, and parasites [18]. The phytochemicals saponin, flavonoid, tannin, reducing sugar, steroid, and terpenoid were present in Allium sativa extracts according to this study. The phytochemical content of the extract of A. sativum revealed that the Alkaloids was found to be the most abundant phytochemical (7.2 %) followed by tannin (4.8 %), saponin (4.3 %) and flavonoids (2.18 %).
Based on the finding of this study, terpenoid is present in the both the extracts. Terpenoids have been found to be useful in the prevention and therapy of several diseases, including cancer. Terpenoids are also known to possess antimicrobial, antifungal, antiparasitic, antiviral, anti-allergenic, antispasmodic, antihyperglycemic, anti-inflammatory and immunomodulatory properties [19]. Flavonoids are also present in the extracts as a potent water-soluble antioxidant and free radical scavenger, which prevent oxidative cell damage and also have strong anticancer activity [20]. It also helps in managing diabetes induced oxidative stress. Steroids are importance in pharmacy as they possess compounds like sex hormones and can be used for drug production [21]. Tannin and saponin were present in the extract. Saponins protect against hypercholesterolemia and antibiotics properties. In addition, it has been found that saponins have antitumor, antioxidant and anti-mutagenic activities and can lower the risk of human cancers by inhibiting the growth of cancer cells [22]. The growth of many fungi, yeast, bacteria and viruses was inhibited by tannins [23]. The finding of this study correlate with the finding of Abaoab et al. [24] which found that clove extract possessed a broad spectrum of antimicrobial activity exhibited for both bacteria and fungi due to presence saponin, tannin, flavonoid and terpenoid. The result of this study on Phytochemistry of Garlic supported the study conducted by Deresse [8] who found that garlic extracts exhibited activity against both gram negative (E. coli, Salmonella sp, and Citrobacter Enterobacter, Pseudomonas Klebsiella) and gram positive (S. aureus, S. pneumonia, streptococcus and Bacillus anthrax) due to presence of some phytochemicals such saponin and tannin.
The results of the present study indicate that the qualitative proximate composition of A. sativum bulb contain carbohydrate, protein, fats, fibre, moisture and ash while the quantitative analysis result was presented as carbohydrate with 66.00%, protein 16.23%, fats 2.44%, crude fibre 03.96%, moisture content 5.52% and ash content 05.85%. This indicated the higher content of carbohydrate when compared to the rest. The higher carbohydrate content may be useful in making A. sativum bulb a good source of energy for the body. The presence of moisture, ash, lipid and protein in A. sativum bulb suggests that it may be useful for body building, prevention of ageing while the high dietary crude fibre content will help in bowel movement. This important nutrients composition in A. sativum bulb provides a justification that the bulb could be used as food supplement. Finding of this study indicated low fat content in A. sativum bulb, and low fat foods are known to reduce cholesterol level [25]. This result was inconformity with that of Harsh et al. [26].
According to the result of this study, the mineral analysis of Moringa leaf extract contained some important essential minerals such as; calcium (23.40%), potassium (10.95%), magnesium (3.90%), zinc (0.44%), phosphorous (9.85%), iron (5.20%) and copper (0.05%). The presence of such minerals in A. sativum bulb could be utilized as a nutritionally valuable and healthy ingredient for food. The mineral elements contained in these spices are very important in human nutrition. Sodium, potassium, calcium and magnesium play a central role in the normal regulation of blood pressure [27]. They could also be valuable in improving immune system and preventing malnutrition related diseases. Mineral elements are required for normal growth, activities of muscles and skeletal development (such as calcium), cellular activity and transport of oxygen (copper and iron), chemical react ion in the body and intestinal absorption (magnesium), fluid balance and nerve transmission (sodium and potassium), as well as the regulation of acid-base balance (phosphorus). Iron is useful in prevent ion of anemia and other related diseases [28]. Manganese plays a role in energy production and in supporting the immune system while zinc is useful for protein synthesis, normal body development and recovery from illness [29].
Conclusion
Based on the findings of the present study, phytochemical constituents, proximate and minerals components of A. sativum bulb were determined. The phytochemical components of A. sativum bulb contain alkaloid, saponins, flavonoids, glycoside, anthraquinones, tannin and terpenoids. The results of the proximate and mineral analyses of the whole leaf indicated the presence of considerable amount of nutrients. The presence of the phytochemicals has authenticated its usefulness by traditional herbalists in ethno medicine and potentials in drug formulation and development. In addition to that, the presence of nutrients proves why A. sativum bulb can be used as food supplement.
References
- Sarker SD, Nahar L (2007) Chemistry for Pharmacy Students General, Organic and Natural Product Chemistry. John Wiley and Sons, England, pp. 283-359.
- World Health Organization (2004) Use of antibacterials outside human medicine and result and antibacterial resistance in humans, WHO 2002.
- Doughari JH (2012) Phytochemicals: Extraction Methods, Basic Structures and Mode of Action as Potential Chemotherapeutic Agents.
- Tiwari R, Das K, Shrivastava DK (2010) Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. Journal of Medicinal Plants Research 4(2): 104-111.
- Onyeagba RA, Ugbogu OC, Okeke CU, Iroakasi O (2004) Studies on the antimicrobial effects of garlic (Allium sativum linn), ginger (Zingiber officinale roscoe) and lime (Citrus aurantifolia linn). Afr J Biotechnol 3(10): 552-554.
- Friesen N, Fritsch RM, Blattner FR (2004) Phylogeny and new intrageneric classification of Allium L. (Alliaceae) based on nuclear ribosomal DNA its sequences. Aliso 22: 372-395.
- Kao SH, Hsu CH, Su SN, Hor WT, Chang WH, et al. (2004) Identification and immunologic characterization of an allergen, alliin-lyase, from garlic (Allium sativum). J Allergy Clin Immunol 113(1): 161-168.
- Deresse D (20100 Antibacterial Effect of Garlic (Allium sativum) on Staphylococcus aureus: An in vitro Study. Asian Journal of Medical Sciences 2(2): 62-65.
- Jaber MA, Al-Mossawi (2007) A Susceptibility of some multiple resistant bacteria to garlic extracts. Afr J Biotechnol 6(6): 771-776.
- Timbo BB, Ross MP, McCarthy PV, Lin CT (2006) Dietary supplements in a national survey: Prevalence of use and reports of adverse events. Am Diet Assoc 106(12): 1966-1974.
- Tsao SM, Yin MC (2001) In vitro antimicrobial activity of four diallyl sulphides occurring naturally in garlic and Chinese leek oil. J Med Microbiol 50(7): 646-649.
- Sofowora A (1993) Medicinal plants and traditional medicine in Africa. Spectrum Books Ltd. (2nd edn), 26-100.
- Trease GE, Evans WC (2002) Phytochemicals. In: Pharmacognosy. (15th edn), Saunders Publishers, London pp. 42-44, 221- 229, 246- 249, 304- 306,331-332, 391-393.
- Adeniyi SA, Orjiekwe CL, Ehiagbonare JE (2009) Determination of alkaloids and oxalates in some selected food samples in Nigeria. African Journal of Biotechnology 8(1): 110-112.
- Udo EJ, Oguwele JA (1986) Laboratory Manual for the analysis of soil, plants and water samples. 3rd edition, Department of crop production, University of IIorin, Kwara State Nigeria 131-152.
- James CS (1995) Analytical Chemistry of Food. Champman and Hall, London, UK, England, pp. 64-65.
- AOAC (1990) Official method of analysis. 4th edition, Association of Officials Analytical Chemists, Washington DC, USA.
- Ibrahim TA, Dada IBO, Adejare RA (2010) Comparative phytochemical properties of crude ethanolic extracts and physicochemical characteristics of essential oils of Myristical fragrans (nutmeg) seeds and Zingiber officinate (ginger) roots. Electronic J Environ Agric Food Chem 9(6): 1110-1116.
- Rabi T, Bishayee A (2009) Terpenoids and breast cancer chemoprevention. Breast Cancer Res Treat 115(2): 223-239.
- Okwu DE (2001) Evaluation of the chemical composition of indigenous spices and flavoring agents. Global Journal of Pure and Applied Sciences 7(3): 455-459.
- Salah N, Miler NJ, Pagange G, Tijburg L, Bolwell GP, et al. (1995) Polyphenolic flavonoids as scavenger of aqueous phase radicals as chain breaking antioxidant. Arch Biochem Broph 322(2): 339-346.
- Roa RR, Babu RM, Rao MRV (1995) Saponins as anti-carcinogens. The Journal of Nutrition 125(3 Suppl): 717-724.
- Prohp TP, Onoagbe IO (2012) Determination of phytochemical composition of the stem bark of triplochiton scleroxylon k. schum. (sterculiaceae). International Journal of Applied Biology and Pharmaceutical Technology 3(2): 68-76.
- Aboaba OO, Ezeh AR, Anabuike CL (2011) Antimicrobial activities of some Nigerian spices on some pathogens. Agriculture and Biology Journal of North America 2(8): 1187-1193.
- Fahey JW (2005) Moringa oleifera: A Review of the medical evidence for its nutritional, therapeutic and prophylactic properties. Trees Life Journal 15(1): 1-15.
- Harsha N, Sridevi V, Lakshmi C, Rani K, Vani S (2013) Phytochemical Analysis of Some Selected Spices. International Journal of Innovative Research in Science, Engineering and Technology 2(11): 6618-6621
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, et al. (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39(1): 44-84.
- Oluemi EA, Akilua AA, Adenuya AA, Adebayo MB (2006) Mineral contents of some commonly consumed Nigerian foods. Science Focus 11: 153-157.
- Muhammad A, Dangoggo SM, Tsafe AI, Itodo AU, Atiku FA (2011) Proximate, minerals and anti-nutritional factors of Gardenia aqualla (Gauden dutse) fruit pulp. In Pakistan Journal of Nutrition10(6): 577- 581.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...