
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Research Article(ISSN: 2637-4609) 
One Step Synthesis of Coumarinfrom Cinnamaldehyde via Cinnamic Acid Intermediate Volume 1 - Issue 1
Made Sudarma*, Siti Laili Zarwati and Made G Darmayanti
- Department of Chemistry, University of Mataram, Indonesia
Received: April 02, 2018; Published: April 13, 2018
*Corresponding author: Made Sudarma, Department of Chemistry, University of Mataram, Indonesia
DOI: 10.32474/AOICS.2018.02.000144
Abstract
The purpose of this study was to develop an efficient method for the synthesis of pharmacologically interesting coumarin (1-benzopyran-2-one) from readily accessed natural products, in particular cinnamaldehyde. One step methodology for the synthesis of coumarin has been developed from cinnamaldehyde. A coumarin was synthesized smoothly via addition of chloro sulfonic acid to cinnamaldehyde. All the products have been confirmed by Thin Layer Chromatography and Gas Chromatography Mass Spectromatry analysis data.
Keywords: One step synthesis; Coumarin; Cinnamaldehyde; Chlorosulfonic acid
Introduction
Coumarin (1-benzopyran-2-one)is an organic compound, belonging to the class of benzopyrones, and is naturally found in many plants.It can be found in cinnamon bark. Coumarin has a wide range of applications such as, as perfumes, cosmetics, and industrial additives. Some of its derivatives have been used as aroma enhancers in tobaccos and certain alcoholic drinks. Moreover, a lot of coumarin compounds as medicinal candidates for drugs with strong pharmacological activity, low toxicity and side effects, fewer drug resistance, high bioavailability, broad spectrum, better curative effects, etc., to treat various types of diseases are being actively studied [1]. Coumarins also have been found to have multi- biological activities such as anti-HIV, anti-tumor, anti-hypertension, anti-arrhythmia, anti-osteoporosis, pain relief, and preventing asthma and antisepsis [2]. While coumarins are only approved for a few remedial uses as pharmaceuticals, it may be noted that during a number of studies, they have also demonstrated some proof of numerous biological activities [3]. Coumarin is known to be moderately toxic to the liver and the kidneys, with an LD50 value of 275mg/kg.
This compound is metabolized differently in mice, rats and humans. Coumarin can be prepared by a number of name reactions with the Perkin reaction between salicylaldehyde and acetic anhydride being a popular example. The Pechmann condensation provides another route to coumarin and its derivatives; as does the Kostanecki acylation which can also be used to produce chromones [4]. An efficient annulation of phenolic acetates with acrylates in the presence of [Rh2(OAc)4] as catalyst and formic acid as reducing agent provides high yield of coumarin derivatives via C-H bond activation [5]. An efficient metal-free tandem acylation/cyclization of alkynoates with aldehydes enables the synthesis of 3-acyl-4- arylcoumarins via addition of acyl radical to alkynes and a C-H bond fictionalization to form two new C-C bonds simultaneously [6]. Arylpropionic acid methyl esters having a MOM-protected hydroxy group at the ortho position underwent hydroarylation with various arylboronic acids in MeOH at ambient temperature in the presence of a catalytic amount of CuOAc, resulting in the formation of 4-arylcoumarins in high yields [7].
Experimental
Materials
The material used included: cinnamon bark, dichloromethane, hexane, methanol, acetate anhydride, ethanol, sodium hydroxide pellet, hydrochloric acid, sodium hydrogen carbonate anhydrous, ethyl acetate, sodium carbonate anhydrous, chlorosulfonic acid, sulphuric acid, analytical thin layer chromatography, silica gel chromatography.
General procedure
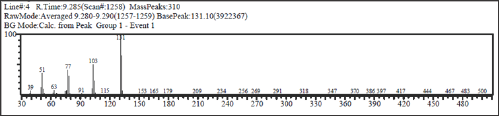
The dried of cinnamon bark was purchased from Suweta traditional market Lombok Barat Regency (250 g) were ground to very small size powder and extracted by maceration with dichloromethane (2x500 mL) for 24 hours at room temperature and stored away from light. Concentration of solution of dichloromethane under reduced pressure by rotary evaporator gave the brownish cinnamon oil (2.35g; 0.94%). The oil was analyzed by Thin Layer Chromatography (TLC) and Gas Chromatography and Mass Spectrometry (GC-MS). TLC analyses showed cinnamaldehydesampel Rf 0.63(eluent, hexane:dichloromethane; 1:1). GC-MS showed cinnamaldehyde 74.13%: M+.132, cal for C9H8O, major fragments: 103 (M+.- CO), 77(benzene ring), and 51.
Isolation of cinnamaldehyde
The cinnamon oil extract (2g) was subjected to silica gel column chromatography (eluent, hexane:dichloromethane; 1:1). Cinnamaldehyde (938mg, 46.91%) was obtained from this separation and confirmed by TLC and GC-MS analysis (Figures 1 & 2).
Figure 1.
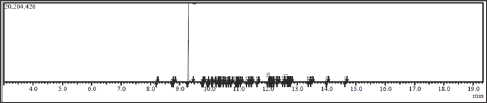
Figure 2.
Synthesis of coumarin
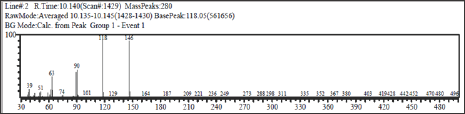
Thisreaction was conducted by adopting method from Sudarma. To stirred solution of cinnamaldehyde (1) (1003 mg, 7.8mmol) in dichloromethane (20mL) was added chlorosulfuric acid (2mL) drop by drop. The solution was stirred at room temperature for 30 min then refluxed for 15 min. The solution was evaporated and water (10mL) then added, basified to pH 8 with 1M sodium hydroxide, then extracted with dichloromethane. The organic phase was dried and evaporated to dryness to give brown gum (685mg). TLC and GC-MS analysis showed coumarine (44.63%).GC-MS: M+.146, cal for C9H6O2.Major fragments: 118 (base peak), and 65 (Figures 3 & 4).
Figure 3.
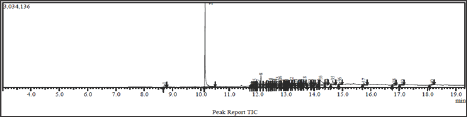
Figure 4.
Results and Discussion
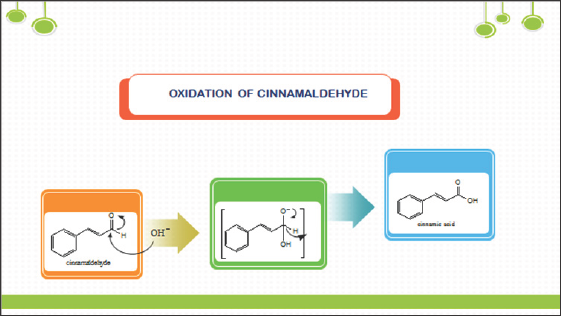
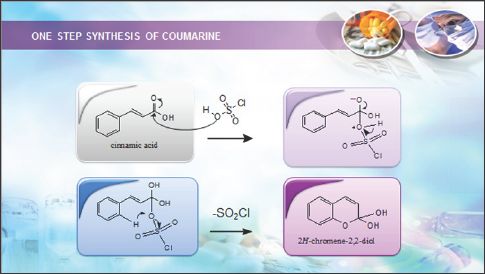
In continuation of our work on transformation of readily accessed natural products such as eugenol and cinnamaldehyde, synthesis of coumarin via addition of chlrosulfonic acid or sulphuric acid to cinnamaldehyde was reported. This works involved isolation of cinnamaldehyde from bark of true cinnamon (Cinnamomumverum) and its transformation to coumarin. Cinnamaldehyde was extracted and isolated from cinnamon oil in good yield (75%). Cinnamaldehydeis an organic compound with the formula C6H5CH=CHCHO as predominately the trans (E) isomer. Occurring naturallyin the bark of cinnamon trees and other species of the genus Cinnamomum like camphor and cassia [8]. Synthesis of coumarin by addition of chlorosulfonic acid to cinnamaldehyde via oxidation reaction to cinnamic acid intermediate (Scheme 1). Simple coumarins are biogenetically derived from shikimic acid, via cinnamic acid [9]. Cinnamaldehyde is oxidized in vivo to cinnamicacid, which excrete in our urine [10]. These mean that on synthesis of coumarin from cinnamaldehyde presumably also via cinnamic acid as an intermediate and involved oxidation reaction. The mild oxidation of cinnamaldehyde should obviously yielded cinnamic acid.In the first step; one mol of water was added in the presence of an acidic catalyst (ClSO3H) to produce a hydrate. Subsequently, the hydrate was oxidized to the cinnamic acid formally by eliminating water. Cyclization ofcinnamic acid to coumarin was presumably initiated by addition of chlorosulfonic acid to its carbonyl (Scheme 2).
Scheme 1: Mechanism of cinnamic acid formation from cinnamaldehyde.
Scheme 2: Synthesis of coumarin from cinnamaldehyde via cinnamic acid.
Conclusion
An efficient method using chlorosulfonic acid reagent was believed to be a suitable method for the synthesis ofcoumarin from cinnamaldehyde [11].
Acknowledgement
The authors thank Indonesian Ministry of Research, Technology, and Higher Education (KementerianRiset, Teknologi, danPendidikan Tinggi) through "HibahBerbasisKompetensi" for financial support.
References
- Benefit from Cinnamon, everything you want to know about cinnamon.
- Maria Joao Matos, Lourdes Santana, Eugenio Uriarte, Orlando A Abreu, Enrique Molina, et al. (2015) Coumarins -An Important Class of Phytochemicals. Agricultural and Biological Sciences.
- Ray Sahelian MD (2016) Coumarin research studies.
- Herbs2000.com
- Coumarin from Wikipedia, the free encyclopedia.
- SK Gadakh, S Dey, A Sudalai (2015) Synthesis of Coumarins. J Org Chem 80: 11544-11550.
- X Mi, C Wang, M Huang, Y Wu (2015) Synthesis of Coumarins. J Org Chem 80: 148-155.
- Y Yamamoto, N Kirai (2008) Synthesis of Coumarins. Org Lett 10: 55135516.
- Cinnamaldehyde, from Wikipedia, the free encyclopedia.
- F Bourgaud, A Hehn, R Larbat, S Doerper, E Gontier, et al. (2006) Biosynthesis of coumarins in plants: A major pathway still to be unravelled for cytochrome P450 enzymes. Phytochem Rev 5(2-3): 293308.
- IM Sudarma, E Yuanita, I W Suana (2013) Markovnikov addition of chlorosulfuric acid to eugenol isolated from clove oil. Indones J Chem 13(2): 181-184.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...















