
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Research Article(ISSN: 2637-4609) 
Improved Enantioselective Enzymatic Synthesis of (S) - Pregabalin Volume 1 - Issue 3
Suresh babu Jayachandra, Madhuresh Sethi*, Vipinkumar Kaushik, Vijayakrishna Ravi, Sanjay Mahajan, Mujahid Sufi Ahmed, Bhairaiah Mara, Gurleen Kaur and Purbita Chakraborty
- R & D, Mylan Laboratories Ltd, India
Received: January 23, 2018; Published: February 15, 2018
Corresponding author: Madhuresh Sethi, R & D, Mylan Laboratories Ltd, Plot No: 31, 32, 33 and 34 A ANRICH Industrial Estate, Andhra Pradesh, India
DOI: 10.32474/AOICS.2018.01.000115
Abstract
The manuscript aims to throw light on the route of synthesis of (3S)-3-(Amino methyl)-5-methylhexanoic acid: (PREGABALIN), which is a cost effective and efficient route. Pregabalin is a drug that is used in the treatment of epilepsy, neuropathic pain, fibromyalgia, and generalized anxiety disorder. This process of synthesis makes use of a liquid enzyme to avoid cost hike and improves the overall chiral purity of the final product accompanied with improved higher yields and purity. The investigational studies performed in this article will help improve the existing processes in certain aspects as discussed above.
Introduction
Pregabalin is available in the market under the brand name of Lyrica among others. It is a drug of choice for the treatment of epilepsy, fibromyalgia, sweeping anxiety disorder and neuropathic pain. It effectively relieves neuropathic pain (pain from damaged nerves) that occurs in your arms, hands, fingers, legs, feet, or toes if you are diabetic or in the area of your rash if you once were affected with shingles (a rash that occurs due to infection with herpes zoster and is also painful) [1]. Pregabalin also finds use in the treatment of fibromyalgia (which is a long-lasting condition causing pain, fatigue, muscle stiffness, tenderness of muscles and difficulty in falling asleep or staying asleep [1]. Pregabalin is used in combination therapy with certain other medications for the treatment of various types of seizures in people who have epilepsy Pregabalin comes under the class of medications that are known as anticonvulsants [2].
In 1990, Pregabalin was synthesized as an anticonvulsant and was invented by medicinal chemist Richard Bruce Silverman at Northwestern University of Chicago, Illinois [3]. The drug received approval in the European Union in the year of 2004. The.com also received FDA approval for its use in treatment of epilepsy, post-therapeutic neuralgia and diabetic neuropathic pain in the December of 2004. It was launched in the.com market as Lyrica in the fall of 2005 [4]. Pregabalin also received approval in the European Union and Russia (not in.com) for treating generalized anxiety disorder [5].
Mechanism of action
Pregabalin's mechanism of action involves reducing the number of pain signals that are sent to the brain by the damaged nerves in the body by binding to certain areas in the brain, which helps reducing nerve pain, seizures, and anxiety (Figure 1) [6]. Pregabalin usually binds to the alpha-2-delta site in the neuron with great affinity and decreases the release of many neurotransmitters that are Ca-dependent (For ex: Glutamate). As it is classified as a GABA (gamma amino butyric acid) analogue, this leads to increase in the levels of GABA in the neurons and subsequent reduction in the associated neuropathic pain, due to its anxiolytic and anti- convulsive effects [7].
Figure 1: Pregabalin structure.
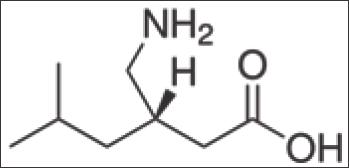
The common side effects of Pregabalin include [8]:
i.Sleepiness or drowsiness
ii. Confusion
iii. Forgetfulness
iv. Poor motor coordination
v. Dry mouth
vi. Vision related problems
vii. Weight gain
There are also some potentially serious side effects of Pregabalin [8]:
i. Angioedema
ii. Drug misuse or addiction
iii. Increased risk of suicide
Also, high doses of Pregabalin consumption over a longer period of time might lead to addiction. However, if prescribed dose is taken, the risk of addiction is low. (3S)-3-(Amino methyl)- 5-methylhexanoic acid (PREGABALIN) is of great importance and the current procedure of its preparation had much scope of improvement and hence it was felt worthwhile to examine an alternate means to prepare this compound (Figure 1).
Material and Methods
Enzymes
TL lipase liquid was purchased from Advanced enzymes.
Chemical reagents
Isovaleraldehyde was purchased from Spectrochem
Analytical Methods
Gas chromatography: Gas chromatographic analysis during the preparation of 2-carbethoxy-5-methyl hex-2-enoic acid ethyl ester and 2-carbethoxy-3-cyano-5-methyl hexanoic acid ethyl ester was performed on Agilent Technologies Gas chromatography instrument using DB-1, (30 m x 0.53mm, 3.0 μm) capillary column. The elution was carried out with nitrogen as carrier gas and the eluents were detected by Flame ionization detector. The retention time was 19.2 min and 20.18 min for the preparation of 2-carbethoxy-5-methyl hex-2-enoic acid ethyl ester and 2- carbethoxy-3-cyano-5-methyl hexanoic acid ethyl ester
Mass Spectrometry: Electron Spray Ionization-Mass spectra (ESI-MS) was measured using Agilent 1100 LC/MSD Trap SL instrument.
NMR spectra: 1H NMR spectra was recorded on a Bruker Avance 300 MHz spectrometer. The spectra was recorded with CDCl3 as solvent and trimethylsilane (TMS) as an internal standard for measuring chemical shifts. A region from 0 - 6 ppm was scanned for all the samples.
Specific Optical Rotation: Specific optical rotation was measured using Perkin-Elmer 243 polarimeter. The specific optical rotation of the compounds were measured at the sample concentration of 1.1% w/v in methanol at 25 °C.
Results and Discussion
The preparation of Pregabalin involves 4 reaction steps which will be studied in this manuscript and will be discussed in detail illustrating some noteworthy observations for an improved process.
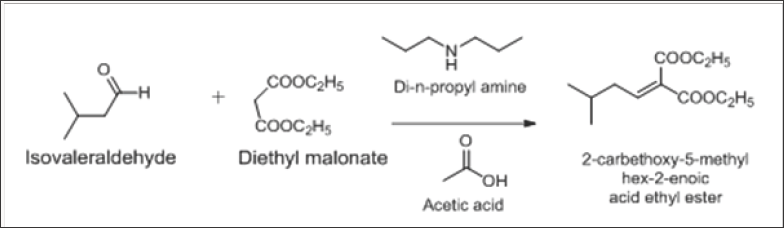
Preparation of 2-carbethoxy-5-methyl hex-2-enoic acid ethyl ester
This reaction involves the well-known naming reaction Claisen condensation between isovaleraldehyde and diethyl malonate in the presence of di-n-propyl amine and acetic acid and refluxed azeotropically to obtain ene-diester. In the reported procedure, the reactants Di-n-propyl amine and acetic acid were added in two lots, one in the starting, and other after 4 hrs. In our process, we added acetic acid and di-n-propyl amine in the starting only and that too 0.05 equivalent of di-n-propyl amine and 0.13 equivalent of acetic acid, in catalytic amount, that results in cost reduction and increased HPLC purity, with no change in the overall yield (Scheme 1).
Scheme 1: Preparation of 2-carbethoxy-5-methyl hex-2-enoic acid ethyl ester.
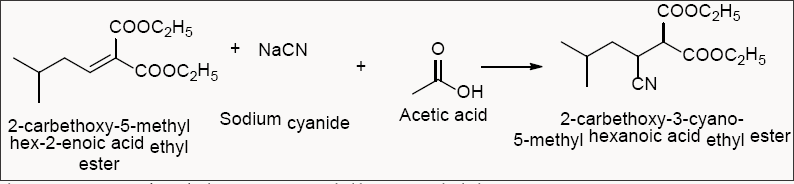
Preparation of 2-carbethoxy-3-cyano-5-methyl hexanoic acid ethyl ester
Sodium Cyanide was used instead of Potassium Cyanide that is recommended in the reported procedure. NaCN is much safer to handle when compared to KCN in industrial use, hence, the change was made. The reported procedure also mentioned distillation of the reaction solvents at 70-1000C but this gave rise to decreased yield of (S)-3-cyano-5-methyl-hexanoic acid ethyl ester. Hence, an alternative was taken of vacuum distillation, which corrected the yield in a positive way (Scheme 2).
Scheme 2: Preparation of 2-carbethoxy-5-methyl hex-2-enoic acid ethyl ester.
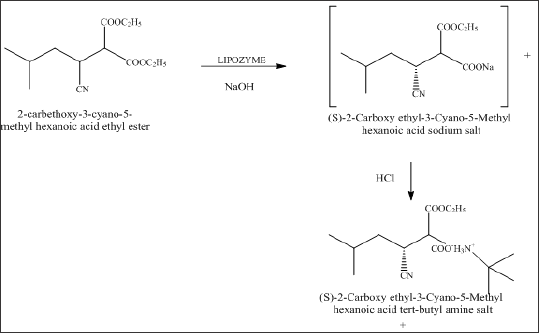
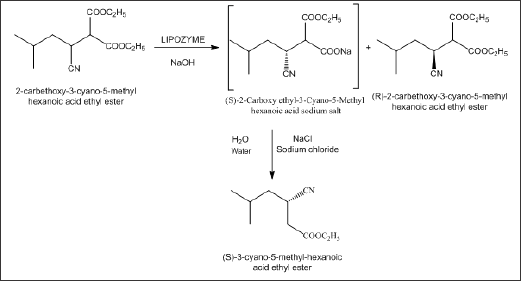
Preparation of (S)-3-cyano-5-methyl-hexanoic acid ethyl ester salt
This reaction involves selective enzymatic hydrolysis using liquid enzyme TL lipase liquid to form sodium salt of 2-Carboxy ethyl-3-Cyano-5-Methyl hexanoic acid, which was then further acidified with Hydrochloric acid to give 2-Carboxy ethyl-3-Cyano- 5-Methyl hexanoic acid, which was then treated with t-butyl amine and S (-)-alpha methyl benzylamine to form its corresponding salt. These salts can be further converted to (S)-3-cyano-5-methyl- hexanoic acid ethyl ester and finally to Pregabalin (Scheme 3).
Scheme 3: Preparation of 2-Carboxy ethyl-3-Cyano-5-Methyl hexanoic acid tert-butyl amine salt.
Some of the other enzymes were screened in our lab, out of which reaction proceeded only with immobilized enzyme but the overall cost is affected because of the high market price of the immobilized enzyme over liquid enzyme. Since no significant change in the overall yield was found, we also proceeded with TL Lipase liquid to have more of an economical process (Table 1).=
The 2-Carboxy ethyl-3-Cyano-5-Methyl hexanoic acid when isolated was found to be unstable and hence, 2-Carboxy ethyl-3- Cyano-5-Methyl hexanoic acid tert-butyl amine salt was prepared in-situ (Table 2). The chirality of the 2-Carboxy ethyl-3-Cyano- 5-Methyl hexanoic acid tert-butyl amine salt was checked by its Specific Optical Rotation (SOR) which was found to be 33.480 (for the R-isomer) and -36.80 (for the S-isomer).
Preparation of (S)-3-cyano-5-methyl-hexanoic acid ethyl ester
Preparation of (S)-3-cyano-5-methyl-hexanoic acid ethyl ester was done in situ in this step, without isolation of 2-Carboxy ethyl- 3-Cyano-5-Methyl hexanoic acid tert-butyl amine salt (Scheme 4).
Table 1: List of enzymes screened.
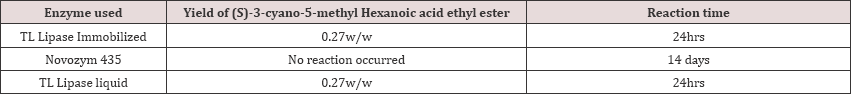
Table 2: Comparison data.

Scheme 4: Preparation of (S)-3-cyano-5-methyl-hexanoic acid ethyl ester.
Preparation of rac-3-cyano-5-methyl-hexanoic acid ethyl ester
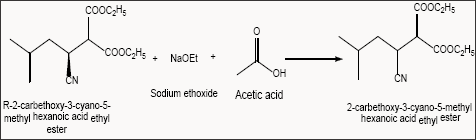
Describes the reaction wherein the unreacted R-2-carbethoxy- 3- cyano-5-methyl hexanoic acid ethyl ester is reversed to form racemic 2-carbethoxy-3-cyano-5-methyl hexanoic acid ethyl ester using sodium ethoxide (Scheme 5).
Preparation of (3S)-3-(Amino methyl)-5-methylhexanoic acid or PREGABALIN
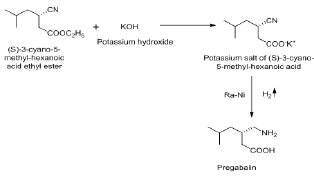
This reaction involves the hydrolysis of ethyl ester, followed by its hydrogenation with Raney Nickel to yield the final product i.e. Pregabalin (Chiral purity >99.9% and purity > 99.8%) (Scheme 6).
Scheme 5: Preparation of racemic 2-carbethoxy-3-cyano-5-methyl hexanoic acid ethyl ester.
Scheme 6: Preparation of (3S)-3-(Amino methyl)-5-methylhexanoic acid or PREGABALIN.
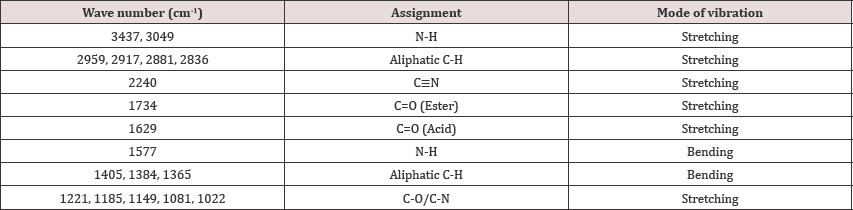
Characterization of the 2-Carboxy ethyl-3-Cyano-5-Methyl a) IR spectroscopy: > Detailed characterization was conducted to confirm the structure of 2-Carboxy ethyl-3- Cyano-5-Methyl hexanoic acid tert-butyl amine salt intermediate of Pregabalin.
a) IR spectroscopy:
Instrument used: Perkin Elmer Spectrum one FTIR spectrometer
Method used: 1% KBr method (Table 3).
Table 3: IR data of the 2-Carboxy ethyl-3-Cyano-5-Methyl hexanoic acid tert-butyl amine salt.
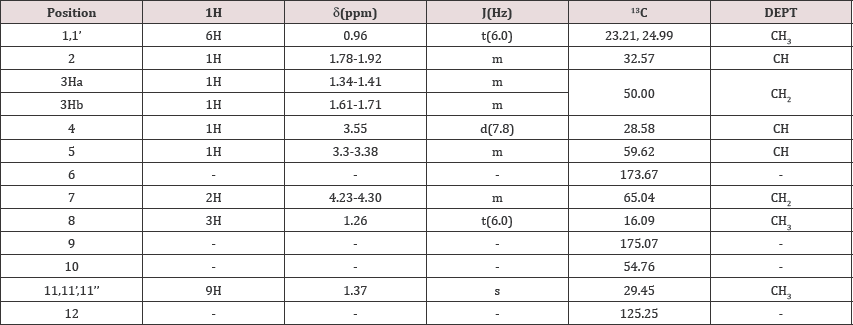
NMR: (Table 4)
Table 4: NMR data of the 2-Carboxy ethyl-3-Cyano-5-Methyl hexanoic acid tert-butyl amine salt.
c) Mass spectroscopy: (Table 5)
Table 5: Describes the mass number of the 2-Carboxy ethyl-3-Cyano-5-Methyl hexanoic acid tert-butyl amine salt.
Optimization of parameters during the preparation of 2-Carboxy ethyl-3-Cyano-5-Methyl hexanoic acid tert-butyl amine salt:
Table 6: pH variations.
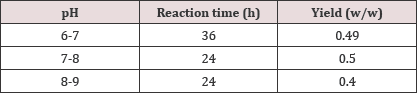
pH variations that were studied are explained in the table below: (Table 6) (Graph 1)
Graph 1: pH vs. Time variation depicted in Graphical representation.
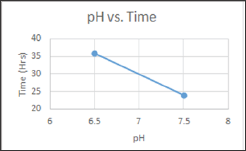
Table 7: Temperature variations.
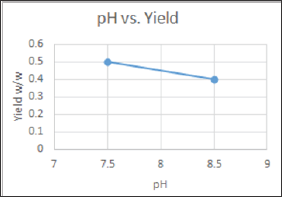
ii. Temperature variations that were studied are explained in the table below: (Table 7) (Graph 2)
Graph 2: pH vs. Yield variation depicted in Graphical representation.
iii. Distillation variations
In the reported procedure, distillation was done at 70-1000C and this led to lesser yield of (S)-3-cyano-5-methyl-hexanoic acid ethyl ester that was prepared in the further reactions. On the other hand, when vacuum distillation was performed, the yield obtained was significantly higher (Table 8).
High vacuum distillation was performed at the end of 2-carbethoxy-5-methyl hex-2-enoic acid ethyl ester since traces of the diethyl malonate impurity would retard the reaction of preparation of (S)-3-cyano-5-methyl-hexanoic acid ethyl ester (Table 9).
Table 8: Purity comparison of 2-carbethoxy-5-methyl hex-2-enoic acid ethyl ester before and after vacuum distillation.

Table 9: Yield differences sighted for simple distillation vs. vacuum distillation.
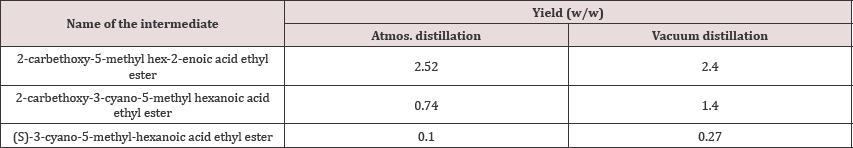
This difference shown in the above table was due to degradation of the compound at higher temperatures during distillation that was performed at 70-1000C. Thus, the yield of the subsequent reactions was also affected. So, it was clear that if vacuum distillation was performed in during the preparation of 2-carbethoxy-5-methyl hex-2-enoic acid ethyl ester and 2-carbethoxy-3-cyano-5-methyl hexanoic acid ethyl ester, yield was higher when compared to distillation at temperature 70-1000C.
Purification techniques employed: At certain stages of the Pregabalin synthesis route, purification techniques were employed to increase the yield of the intermediate products of the subsequent stages.
i. Fractional distillation of 2-carbethoxy-5-methyl hex-2- enoic acid ethyl ester was done that resulted in the increase in yield of (S)-3-cyano-5-methyl-hexanoic acid ethyl ester
ii. Column purification of 2-carbethoxy-3-cyano-5-methyl hexanoic acid ethyl ester was also done which in turn increased the purity of (S)-3-cyano-5-methyl-hexanoic acid ethyl ester and 2-Carboxy ethyl-3-Cyano-5-Methyl hexanoic acid ter-butyl amine salt.
Conclusion
Modifications done in the preparation of 2-carbethoxy-5- methyl hex-2-enoic acid ethyl ester indirectly helped improve the yield of (S)-3-cyano-5-methyl-hexanoic acid ethyl ester, during the synthesis of (3S)-3-(Amino methyl)-5-methylhexanoic acid. Higher purity and cheaper cost of the product, are the primary benefits that can be drawn from this improved synthetic procedure of (3S)-3- (Amino methyl)-5-methylhexanoic acid. Time saving is yet another advantage of the proposed method of synthesis. Hence, it can be concluded by saying that the proposed method in this manuscript will help modify the existing synthesis of (3S)-3-(Amino methyl)-5- methylhexanoic acid and give way to better process.
Acknowledgement
Our group would like to thank the Department of Scientific and Industrial Research India, Dr. Hari Babu (COO Mylan), Sanjeev Sethi (Chief Scientific Officer Mylan Inc); Dr Abhijit Deshmukh (Head of Global OSD Scientific Affairs); Dr Yasir Rawjee (Head - Global API), Dr Sureshbabu Jayachandra (Head of Chemical Research), Dr. Suryanarayana Mulukutla (Head Analytical Dept MLL API R & D) as well as analytical development team of Mylan Laboratories Limited for their encouragement and support. We would also like to thank Dr Narahari Ambati (AGC- India IP) & his Intellectual property team for their support.
References
- Frampton JE (2014) Pregabalin: A Review of its Use in Adults with Generalized Anxiety Disorder. CNS Drugs 28(9): 835-854.
- Hamandi K, Josemir WS (2006) Pregabalin: A new antiepileptic drug for refractory epilepsy. Seizure 15(2): 73-78.
- Derek L (2015) Getting To Lyrica. In The Pipeline.
- Dworkin RH, Kirkpatrick P (2005) Fresh from the Pipeline: Pregabalin. Nature Reviews Drug Discovery. 4(6): 455-456.
- Bandelow B, Wedekind D, Leon T (2007) Pregabalin for the treatment of generalized anxiety disorder: a novel pharmacologic intervention. Expert Review of Neurotherapeutics 7(7): 769-81.
- Ryan Patel, Anthony H Dickenson (2016) Mechanisms of the gabapentinoids and a2S-1 calcium channel subunit in neuropathic pain. Pharmacology Res Perspectives 4(2): e00205.
- (2015) Pregabalin. The American Society of Health-System Pharmacists.
- (2011) Medication Guide.com Food and Drug Administration.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...












