Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Research Article(ISSN: 2637-4609) 
GS-MS Study, Antimicrobial and Antioxidant Activity of Fixed Oil from Ximenia Americana L. Seeds Volume 5 - Issue 4
Tuhami Elzein Hagr1, Mahdi Abd elmageed2, Salah Eldeen H Abdlrazig3, Awad Salim Holi4 and Hatim M Y Hamadnalla5*
- 1Department of Chemistry and Industrial Chemistry, College of Applied and Industrial Sciences, University of Bahri, Africa
- 2Department of Biology and Technology, College of Applied and Industrial Sciences, University of Bahri, Africa
- 3,4Department of Chemistry, University of Western Kurdofan, Alnhud Faculty of Education, Africa
- 5Department of Biochemistry College of Applied and Industrial Sciences. University of Bahri. Africa
Received:August 10, 2021 Published:August 23, 2021
*Corresponding author:Hatim M Y Hamadnalla, Department of Biochemistry College of Applied and Industrial Sciences. University of Bahri. Sudan, Africa
DOI: 10.32474/AOICS.2021.05.000217
Abstract
The aim of the present study is to investigate the chemical constituents of the Essential Oil from Ximenia americana L. plant Seeds and to evaluate its antioxidant potential and antibacterial activity. Using Soxhlet method to extract the essential oil from Ximenia americana L. plant Seeds. The chemical constituents of Ximenia americana Oil were identified and quantified by GC-MS, where paper disc diffusion assay was employed to evaluate the antibacterial activity and Antioxidant activities were evaluated using 1,1-diphenyl-2-picryl hydrazyl (DPPH) radical scavenging activity Twenty-three components has been identified. Ten of them are major namely 9-Octadecenoic acid (Z)-, methyl ester (32.40% ), Methyl octadeca-9-yn-11-trans- enoate (13.08%) , 9,12-Octadecadiynoic acid, methyl ester (2.69%) , 15-Tetracosenoic acid, methyl ester, (Z)- (4.99 %) , , 10-Nonadecenoic acid, methyl ester (6.81% ), Hexacosanoic acid, methyl ester (4.12%), Tetracosanoic acid, methyl ester (3.02%), Cyclopropaneoctanoic acid, 2-octyl-, methyl ester (15.93%), Octacosanoic acid, methyl ester2.38%) and cis-10-Nonadecenoic acid, methyl ester (7.32% ). the antibacterial showed showed low inhibitory effect against Pseudomonas aeruginosa (9mm), Bacillus subtilis (10mm), albicans (10mm) and Escherichia coli (11mm) and Candida albicans. The DPPH assay showed moderate antioxidant potential (49, 0.01 compared with standard 91± 0.01.
Keywords: GC-MS Analysis; Antibacterial and Antioxidant Activities
Introduction
Flora of Sudan is rich with 3140 species of flowering plants belonging to 170 family and 1280 genera [1], including the progenitors of many important food crop of today. Some medicinal species were well known as the basis of European herbal medicine. Many of these plants were threatened where other disappeared. Ximenia americana L. plant (X.a) belong to Family Olacaceae, (known as Umedica), is spiny shrub or tree up to 6m [2]. The fruits of the plant up to 3cm long, light green turning to yellow, orange, or red onripening, containing a small embryo; and they have up to 60% oil [3]. Phytochemical screening of the extract of the plant revealed the present of saponins, cyanogenic glycosides, flavonoids, and tannins [4], also the extracts of (X.a) showed an antioxidant activity [5]. In Sudan, the different parts of the plant extraction are used in folk medicines as: leaves and twigs used to treatment of fever, colds, mouthwash for toothaches, laxative, and eye lotion [6]. The extract of the leaves used for headaches and poison antidote, where roots used for skin aches, headaches, leprosy, hemorrhoids, sexually transmitted diseases, guinea worm, sleeping sickness and as poison antidote [7]. The bark and fruits applied skin ulcers, treating habitual constipation, used as vermifuge and it’s eaten [8]. Ximenia americana L. used for treatment of irregular menstruation,rheumatism and cancer [9], and for treatment diarrhea [10]. Investigation showed that the constituents of (X.a) have showed several biological activities such as antimicrobial, antifungal, anticancer and antioxidant, and the antimicrobial activity of the extract of (X.a) appeared to be due to the presence of secondary metabolites such phenol, terpenes, tannins, and glycosides [11,12 )
Materials
Plant material
Ximenia americana fruits were collected from forest around Almoglad City-West Kordofan state – Sudan - on January 2019. The plant was authentically by Dr. Ahmed Suliman - Gum and Forest Products Research Center -West Kordofan University-Alnohud- Sudan.
Extraction of oil
100 g of the seeds were ground into fine powder. Powdered seeds were extracted with n-hexane using Soxhlet extractor for six hours. The volume of hexane was reduced under reduced pressure. The oil of Ximenia americana (L) was obtained by evaporating the reduced hexane by air drying in a steady current. The oil was kept in a refrigerator for further manipulation.
GC /MS method
The qualitative and quantitative analysis of the sample was carried out by using GC MS technique model ( GC /MS-QP2010- Ultra) from japan “Simadzu Company ,with capillary column (Rtx- 5ms-30 ×0.25 mm ×0.25 μm ).The sample was injected by using split mode ,helium as the carrier gas passed with flow rate 1.61 ml/min, the temperature program was started from 60 C with rate 10 C /min to 300 c as final temperature degree , the injection port temperature was 300c, the ion source temperature was 200 c and the interface temperature was 250 c .The sample was analyzed by using scan mode in the range of m/z 40 – 550 charge to ratio .Identification of component for the sample was achieved by comparing their retention times and mass fragmentation patent with those available in the library, the National Institute of Standards and Technology (NIST),results were recorded.
Antimicrobial assay
Disc diffusion method: The paper disc diffusion method was used to screen the antibacterial activity of plant extracts and performed by using Mueller Hinton agar (MHA). The experiment was carried out according to the National Committee for Clinical Laboratory Standards Guidelines [13]. Bacterial suspension was diluted with sterile physiological solution to 108cfu/ ml (turbidity = McFarland standard 0.5). One hundred microliters of bacterial suspension were swabbed uniformly on surface of MHA and the inoculum was allowed to dry for 5 minutes. Sterilized filter paper discs (Whatman No.1, 6 mm in diameter) were placed on the surface of the MHA and soaked with 20 μl of a solution of each plant extracts. The inoculated plates were incubated at 37°C for 24 h in the inverted position. The diameters (mm) of the inhibition zones were measured.
DPPH radical scavenging assay
The DPPH radical scavenging was determined according to the method of [14] with some modification. In 96 –well plate, were allowed to react with 2, 2 Di (4-tert-octylphenyl)-1-picrylhydrazyl stable free radical (DPPH) for half an hour at 37 C. The concentration of DPPH was kept as (300μL M). The test sample was dissolved in DMSO while DPPH was prepared in ethanol. After incubation, decrease in absorbance was measured at 517 nm using mutilate reader spectrophotometer. Percentage radical scavenging activity by sample was determine in comparison with DMSO treated control group all testes and analysis were run in triplicate.
Results and discussion
Constituents of Ximenia americana L seed oil
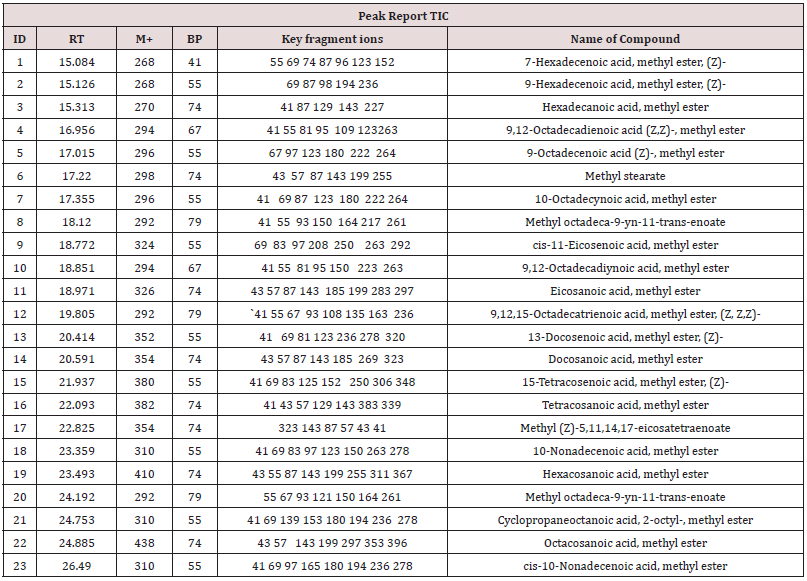
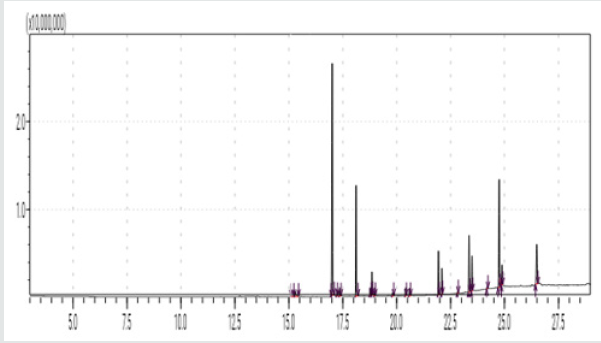
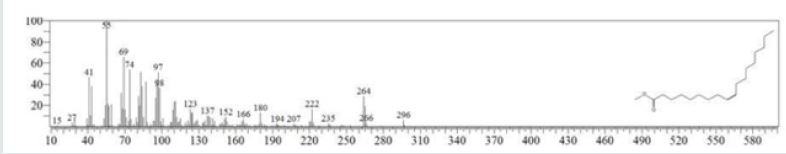
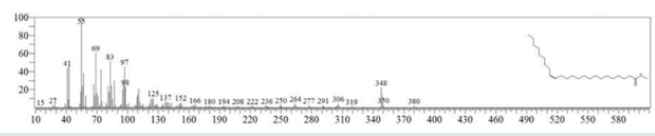
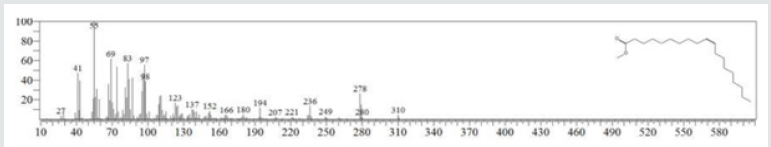
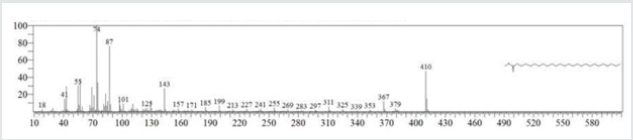
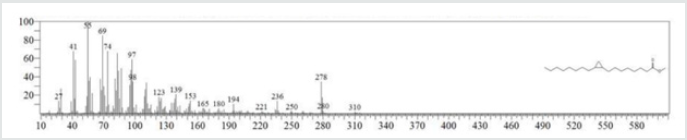
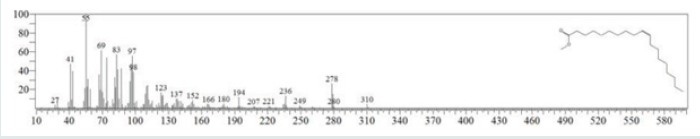
The typical GC chromatogram of oil extracted from Ximena Americana L. Seeds revealed the presence of twenty-three components was presented in (Figure 1) and (Table 1). Ten of them are major namely 9-Octadecenoic acid (Z)-, methyl ester (32.40% ), Methyl octadeca-9-yn-11-trans-enoate (13.08%) , 9,12-Octadecadiynoic acid, methyl ester (2.69%) , 15-Tetracosenoic acid, methyl ester, (Z)- (4.99 %) , , 10-Nonadecenoic acid, methyl ester (6.81% ), Hexacosanoic acid, methyl ester (4.12%), Tetracosanoic acid, methyl ester (3.02%), Cyclopropaneoctanoic acid, 2-octyl-, methyl ester (15.93%), Octacosanoic acid, methyl ester2.38%) and cis-10-Nonadecenoic acid, methyl ester (7.32% ). The peak appeared at 17.015 min with area (32.40%) and has MS ions m/z 337 [M] + correspond to formula C19H36O2 (Figure 2) as well and 55 (base peak) which agree with 9-Octadecenoic acid (Z)- , methyl ester. The peak of Methyl octadeca-9-yn-11-trans- enoate is appeared at 18.120 min with area (13.08%) and has MS ions m/z 346 [M] + correspond to formula C19H38O2 Figure 3 Figure 4 shows the mass spectrum for 9,12-Octadecadiynoic acid, methyl ester which appeared at 18.851 min in total ion chromatogram with area (2.69%) this compound has MS ions m/z 458 [M] + correspond to formula C19H30O2 .The peak at R.T. 21.937 min with Area (4.99 %) on GC chromatogram, produced molecular ion peaks m/z at 426 [M] + corresponded to formula C25H48O2 in it MS spectra Figure 5, which are in agreement with 15-Tetracosenoic acid, There are another peaks appeared at 22.093 , 23.359 , 23.493 , 24.753 , 24.885, and 26.490 mint with Area (6.81, 4.18,3.02,15.93,2.38 and 7.32 %) respectively (Figures 6- 11) produced molecular ion peaks m/z at 381 ,510,488, 530,455 and 536 [M]+ corresponded to formula C19H36O2 , C27H54O2 , C25H50O2, C20H38O2 , C29H58O2 and C20H38O2 in their MS spectra (Figures 5-7) in addition these compounds identified to be 10-Nonadecenoic acid, methyl ester , Hexacosanoic acid, methyl ester, Tetracosanoic acid, methyl ester, Cyclopropaneoctanoic acid, 2-octyl-, methyl ester , Octacosanoic acid, methyl ester and cis-10-Nonadecenoic acid, methyl ester. The present work reveals that the extract of Ximenia americana L showed moderately activity against microorganisms Are given in (Table 2) and The DPPH assay showed antioxidant potential (49±0.01) compared with standard (91±0.01). Are given in (Table 3) is a good source of antioxidants due to the presence of fatty acids compounds, also is a potential source of natural antibacterial, and justify its uses in folkloric medicines. In conclusion, the results showed that the Oïl of Ximenia americana L a potential source of natural antibacterial, antioxidant potential and justify its uses in folkloric medicines. Our results showed that the Fixed oil extracted from Ximenia americana L seeds rich with various fatty acids derivatives, phenolic compounds, Steroidal derivatives and pentacyclic triterpenes. The existence of these bioactive chemical compounds proved the use of this plant for various ailments by traditional medical practitioners. The Ximenia americana L seeds fixed oil have significant antibacterial and antioxidant capacity, suggesting using in flavoring agent, food industry and medicinal purposes.
(Ba = Bacillus subtilis, Ec = Escherichia coli, Pa = Pseudomonas aeruginosa, Ca = Candida albicans, Bs = Bacillus subtilis)
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- Elamin, HM (1990) Trees and shrubs of Sudan. Ithaca press Exeter, UK pp.491.
- Sacande M, Vautier H (2006) Ximenia americana, Seed, Leaf pp. 112.
- Maundu PM, Nugugi GW, Kabuye CHS (1999) Traditional Food Plants of Kenya, Kenya Resources Centre for Indigenous Knowledge. National Museums of Kenya pp. 288.
- Ogunleye DS, Ibitoye SF (2003) Stadies of antimicrobial activity and chemical constituents of Ximenia americana. Trop J Pharm Res 2(2): 239-241.
- Atawodi SE (2005) Antioxidant potential of African medicinal plants, African Journal of Biotechnology 4(2): 128-133.
- Omer MEA, Ali MAZ (1998) Sudanese plants used in folkloric medicine screening for antimicrobial activity, Fitoterapia 69: 542-545.
- Teo SP (1997) Root hemi parasitism in Malayan Olacaceae. Garden Bull Singapore 49(4): 7-13.
- Niemi L, Wennstron A, Ericson L (2005) Insect feeding references and plant phenolic glycosides in the system Conioctena Linnaeana salix triandra, Entomologia Experimentalism et Applicator 115: 61-66.
- Chhabra SC, Viso FC (1990) A Survey of the Medical Plants Eastern Tanzania for Alkaloids, Flavonoids, Saponins and Tannins. Fitoterapia 61(4): 307-316.
- Mathable MC, Nikova RV, Lall N, Nyazema, NZ (2006) Antibacterial activities of medicinal plants used for treatment of diarrhea in Limpopop province, South Africa. Journal of Ethno pharmacology, 105(1-2): 286-293.
- James DB, Abu EA, Wurochekke AU, Orgi GN (2007) Phytochemical and antimicrobial Investigation of the Aqueous and Methanolic Extraction of Ximenia americana. Journal of Medical Science 7(2): 284-288.
- Kokate CK (2000) Practical Pharmacognosy. Vallabh Prakashan, Delhi, India pp. 107‐111.
- National Committee for Clinical Laboratory Standards (NCCLS) (1999) Performance standards for antimicrobial susceptibility testing; ninth Informational supplement. Wayne, Pensilvania document M100-S9, Vol.19.
- Schaller H (2003) Progress in Lipid Research 42(3): 163.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...