
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Mini Review(ISSN: 2637-4609) 
Benzotriazole: A Versatile Synthetic Auxiliary Volume 2 - Issue 1
Siva S Panda*
- Department of Chemistry & Physics, Augusta University, USA
Received: March 01, 2018; Published: March 09, 2018
*Corresponding author: Siva S Panda, Department of Chemistry & Physics, Augusta University, Augusta, GA 30912, USA
DOI: 10.32474/AOICS.2018.02.000129
Introduction
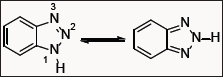
Benzotriazole (BtH) is particularly versatile synthetic auxiliary because of its attractive properties. Benzotriazole can be easily inserted into molecules and equally can also act as a good leaving group. This molecule is a weak acid (pKa 8.2) as well as weak base (pKa < 0) and because of this acid-base property of benzotriazole. This molecule also shows not only electron donating but also electron attracting ability, which leads to various synthetic applications. In 1980, benzotriazole was first reported as synthetic auxiliary in organic chemistry [1]. Since then benzotriazole is used in the construction of various monocyclic and bicyclic heterocyclic compounds which are difficult to prepare by other methods [2-5].
Benzotriazole in heterocyclic synthesis
Heterocycles are important class of compounds. Benzotriazole extensively used for the synthesis of various heterocycles. 1-Aza- 1,3-bis(triphenyl phosphoranylidene)propane synthesized from N-((1H-benzo[d][1,2,3]triazol-1-yl)methyl)-1,1,1-triphenyH5- phosphanimine and methylene triphenyl phosphorane in presence of n-butyllithium, used as potential building block for the synthesis of heterocycles like 3H-benzo[c] azepine and 2,3-disubstituted pyrroles [6].
Figure 1:
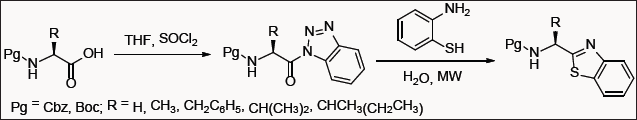
2-Substituted benzothiazoles are well known for their biological properties with numerous accounts listing their synthesis [7]. Recently, benzotriazole technology was used in water as a one-pot approach to synthesize 2-peptidyl benzothiazole in excellent yield without any detectable racemization [8]. The effectiveness of this approach relies in the fact that it neither required any additional reagents nor catalyst for completion (Figure 1-3).
Figure 2:

Figure 3:
Benzotriazole in Acylation, Aroylation and Substitution Reactions
Benzotriazole and its derivatives plays important role in various reactions like acylation, aroylation and substitution reactions [2,9]. Substitution of hydroxyl group with chloride group of the sterically and electronically hindered alcohol of carbohydrates involves harsh reaction conditions and expensive reagents. Furthermore, the formation of halides from nucleofugal groups leads in the formation of unsaturated products and also chances of losing chirality due to harsh reaction condition. Saxena et. al. reported benzotriazole sulfonate (prepared in-situ from benzotriazole and thionylchloride)as potential reagent for the one pot conversion for the sterically and electronically hindered alcohol of carbohydrates into their corresponding chloride derivatives in excellent yield [9].
Benzotriazole in Phosphorous Chemistry
Organophosphorus compounds are important class of compounds. Dialkyl- and diaryl halo phosphates are generally used as building block for the synthesis of organophosphorous compounds. However these building blocks suffer from lack of stability, particularly to hydrolysis and also required toxic reagents to synthesize them. Katritzky group synthesized and demonstrated the use of 1Hbenzo[d] [1,2,3]triazol-1-yl-1-phosphonates as a potential phosphonylation Reagents, which is quite stable than chloro derivatives [10].
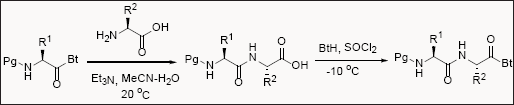
Benzotriazole in cyclic peptide synthesis
N-acylbenzotriazoles are superior acylating agent with various advantages over traditional acid chlorides. N-acylbenzotriazoles are quite stable and utilized in peptide chemistry and showed better applications in compared to other peptide coupling reagents. Unprotected amino acids couple with N-protected aminoacylbenzotriazoles in aqueous acetonitrile to deliver enantiopure dipeptides in good yield. For the synthesis of tripeptide, the carboxylic group of dipeptide is activated by benzotriazole in presence of thionylchloride followed by treatment with unprotected amino acids resulted in desired tripeptide in excellent yields. Hence, benzotriazole assisted chemistry was successfully utilized to synthesize up heptapeptides in solution phase without any detectable loss of absolute configuration [11-13].
Benzotriazole in Peptide Synthesis
Peptidomimetics are special class of compounds which are mimic to natural peptides but resistant to enzymatic hydrolysis. Several peptidomimetics like aminoxopeptides, depsipeptides, azapeptides, oxyazapeptides and hydrazine peptides were synthesized [14-18].
Benzotriazole in Peptidomimetic Synthesis
Figure 4:
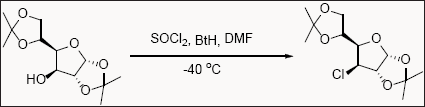
Figure 5:
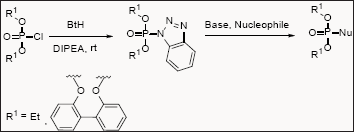
Figure 6:
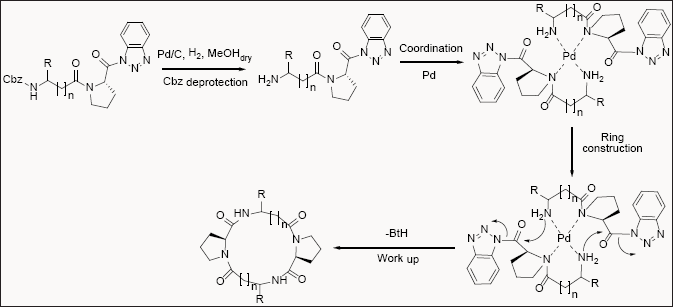
Cyclic peptides are unique class of compounds. The synthesis of cyclic peptides is often challenging. Benzotriazole methodology was extended to use in the synthesis of various cyclic peptides via dimerization macrocyclization approach [19] Figure [4-6].
N-Cbz-dipeptidoyl benzotriazolides are forced to dimerization/ cyclization to form both C2 symmetrical and unsymmetrical cyclic tetrapeptides by utilizing a Pd-assisted tandem deprotection/ cyclization reaction. However synthesis of these types of cyclic- peptides is not efficiently prepared by other reported methods [20].
Conclusion
Benzotriazole offers wide range of application as a potential synthetic auxiliary for various synthetic applications Figure 7 & 8.
Figure 7:
Figure 8:
References
- Katritzky AR, Rachwal S (1987) B J Chem Soc, Perkin Trans 1: 805.
- Hall CD, Panda SS (2016) Adv Heterocyclic Chem 1: 119.
- Katritzky AR, Rachwal S (2010) Synthesis of heterocycles mediated by benzotriazole, Monocyclic systems. Chem Rev 110: 1564-1610.
- Katritzky AR, Rachwal S (2011) Synthesis of Heterocycles Mediated by Benzotriazole, Bicyclic Systems Chem Rev 111: 7063-7120.
- Zorc B, Dzolić, ZR, Butula I (2012) Benzotriazole as a Synthetic Auxiliary Croat Chem Actal 85: 595.
- Katritzky AR, Jiang J, Steel P (1994) J J Org Chem 59: 4551.
- Prajapati NP, Vekariya RH, Borad MA, Patel (2014) H D RSC Adv 4: 60176-60208.
- Panda SS, Ibrahim MA, Oliferenko AA, Asiri AM, Katritzky AR, et al. (2013) Green Chem 15: 2709.
- Azad CS, Saxena A K (2013) Tetrahedron 69: 2608.
- Panmand DS, Tiwari AD, Panda SS, Monbaliu JCM, Beagle LK, et al. (2014) Tet Lett 55: 5898.
- Panda SS, Hall CD, Scriven E, Katritzky AR (2013) Aldrichimica Acta 46: 43.
- Bajaj K, Panda SS, El Nachef C, Katritzky A R (2012) Chem Bio Drug Design 80: 17.
- Abdelmajeid A, Tala SR, Amine MS, Katritzky AR (2011) Synthesis 18: 2995.
- Avan I, Hall CD, Katritzky AR (2014) Peptidomimetics via modifications of amino acids and peptide bonds. Chem Soc Rev 43: 3575-3594.
- Avan I, Tala SR, Steel PJ, Katritzky AR (2011) J Org Chem 76: 4884.
- Biswas S, AboDya NE, Oliferenko A, Khiabani A, Steel PJ, et al. (2013) J Org Chem 78: 8502.
- AboDya N E, Biswas S, Basak A, Avan I, Alamry KA, et al. (2013) Benzotriazole-Mediated Synthesis of Aza-peptides: En Route to an Aza- Leuenkephalin Analogue. J Org Chem 78: 3541.
- Panda SS, El Nachef C, Bajaj K, Katritzky AR (2013) Eur J Org Chem pp. 4156.
- Ha K, Lebedyeva I, Hamedzadeh S, Li Z, Quinones R, Pillai GG (2014) R Chem Eur J 20: 4874.
- White CJ, Yudin AK (2011) Contemporary strategies for peptide macrocyclization. Nat Chem 3: 509.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...















