Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5945
Research Article(ISSN: 2638-5945) 
Use Of Surveillance, Epidemiology, And End Results (SEER)-Medicare Data to Characterize the Demographics, Disease Characteristics, Comorbidities, And Survival of Elderly (≥65 Years) Patients with Human Epidermal Growth Factor Receptor-2-Positive Breast Cancer as Primary Cancer in the United States Volume 4 - Issue 5
Nora Tu1*, Zahidul Islam1, Mackenzie Henderson1,2 and Maribel Salas1,3
- 1Global Epidemiology Department, Daiichi Sankyo, USA
- 2Rutgers Institute for Pharmaceutical Industry Fellowships, Rutgers University, USA
- 3Center for Clinical Epidemiology and Biostatistics (CCEB)/Center for Pharmacoepidemiology Research and Training (CPeRT), University of Pennsylvania Perelman School of Medicine, USA
Received:August 5, 2021 Published: August 19, 2021
Corresponding author: Nora Tu, Global Epidemiology Department, Daiichi Sankyo, 211 Mount Airy Road, Basking Ridge, NJ, USA
DOI: 10.32474/OAJOM.2021.04.000198
Abstract
Purpose: A retrospective cohort analysis to investigate comorbidities and 5-year survival in elderly patients (≥65 years) with human epidermal growth factor receptor-2-positive (HER2-positive) breast cancer (BC) using data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare program
Methods: Female patients aged ≥65 years with a HER2-positive BC diagnosis as the first cancer diagnosis (between 1/1/2010– 12/31/2015) were eligible. Comorbidities and 5-year survival of HER2-positive BC patients (irrespective of stage) were compared with age/race-matched non-cancer controls. Survival data (time-to-death) were unadjusted and adjusted by comorbidities and region.
Results: Data from 8978 HER2-positive BC patients and 8978 controls were included. Mean age was 77.1 years (range: 66.0– 102.8 years). Most cancers were Stage I (35.1%) or II (33.2%) at diagnosis. Thirteen/24 predefined comorbidities were significantly more prevalent in HER2-positive BC patients versus controls (P < 0.05). The most prevalent comorbidities in HER2-positive BC patients were anemia, hypertension, and arthritis. Generally, comorbidities were more likely to be diagnosed before HER2-positive BC diagnosis. The estimated probability of 5-year survival was 69.7% (95% CI: 68.6%, 70.7%) for BC patients compared to 82.4% (95% CI: 81.5%, 83.3%) for controls. The risk of dying was higher among HER2-positive BC patients compared to non-cancer controls, with an unadjusted hazard ratio (HR) of 1.843 (95% CI 1.734,1.960) and adjusted HR of 1.934 (95% CI 1.819,2.057).
Conclusions: Many comorbidities were more prevalent in elderly patients with HER2-positive BC compared with controls, though few were more prevalent in controls. Comorbidities were generally more likely to be diagnosed prior to HER2-positive BC diagnosis. Mortality risk in patients with HER2-positive BC was higher than controls even after adjusting by comorbidities, suggesting that other factors are involved in the survival of elderly patients with HER2-positive BC. The clinical relevance of comorbidities in HER2+ BC patients and controls requires further study.
Keywords: Comorbidity; Elderly; HER2-positive breast cancer; SEER-Medicare; Survival
Abbreviations: 
Introduction
A number of factors can negatively impact on health outcomes in patients with cancer, including age (elderly), socioeconomic status (low), cancer stage (late advanced), and comorbidities (many/ severe). Comorbidities increase with age and represent additional challenges in the management of oncology patients [1]. In elderly patients with breast cancer (BC), the presence of comorbidities may negatively impact health outcomes including survival [2,3]. Comorbidities such as prior myocardial infarction, liver disease, and chronic renal failure are known to have a detrimental impact on survival in elderly patients with BC [2]. Human epidermal growth factor receptor-2-positive (HER2-positive) BC is an aggressive form of BC caused by the over production of the HER2 protein [4- 6] In recent years, the prognosis and outcomes of HER2-positive BC patients have improved considerably since the introduction of HER2-targeted therapies such as trastuzumab, pertuzumab, ado-trastuzumab emtansine, trastuzumab deruxtecan, neratinib, lapatinib, and tucatinib [7-21]. Furthermore, clinical trials are underway using cyclin-dependent kinase 4/6 inhibitor-targeted therapies (palbociclib, abemaciclib, and ribociclib) in patients with advanced HER2-positive BC, with the hope that such drugs could provide a further treatment option for patients with advanced/ metastatic disease [22]. In 2010, the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute began collecting HER2-status data for BC patients registered in SEER (SEER-derived HER2+ recode 2010+) [23]. The data from SEER are from registries, which collect clinical, demographic, and cause-of-death information from people with cancer, and these data are linked to Medicare claims for covered healthcare services from the time a person is eligible for Medicare until their death (SEER-Medicare). A retrospective cohort analysis was conducted to investigate comorbidities, BC disease characteristics, demographics, and overall survival (OS) in elderly patients (≥65 years) with HER2- positive BC using SEER-Medicare data from 2010-2015. At the time of this analysis, there were limited published data on comorbidities and health outcomes in elderly patients with HER2-positive BC.
Materials and Methods
Patient population
This retrospective cohort analysis used data derived from 18 registries that form or formed part of the SEER-Medicare database based on the year they entered the SEER-Medicare program: (Connecticut [1973+]; Detroit [1973+]; Hawaii [1973+]; Iowa [1973+]; New Mexico [1973+]; Seattle-Puget sound [1974+]; Utah [1973+]; Kentucky [2001+]; Louisiana [2001+]; New Jersey [2000+], San Francisco-Oakland [1973+]; Los Angeles [1992+]; San Jose- Monterey [1992+]; Greater California (excluding SF, Los Angeles, and SJ) [2001+]; Atlanta [1974+]; Rural Georgia [1974+]; Greater Georgia [excluding AT and RG] [2010+], and Alaska [1999+]). Eligible patients for this study were female, aged ≥65 years, with a HER2-positive BC diagnosis as the first cancer diagnosis, which was first confirmed (first diagnosis date) between January 1, 2010, and December 31, 2015. Patients were also required to have been continuously enrolled in both Medicare Part A (hospital, skillednursing facility, hospice, and some home healthcare) and Part B (physician and outpatient services) for 12 months prior to their index date (the first diagnosis date was used as the index date for the purpose of the analysis). Patients were excluded if they were enrolled in a Health Maintenance Organization (HMO) plan and/or if the reason for their original enrollment was due to disability or end-stage renal disease.
Matched Control Population
The control population were non-cancer subjects matched by age and race to the HER2-positive BC study group. Non-cancer controls were drawn from a 5% random sample of the Medicare population who resided in SEER areas and who did not have any cancer diagnosis at the time of the survey (data accessed via the Summarized Denominator file). In addition, non-cancer controls had to be female, aged ≥65 years in 2010, and still alive in 2010 when the data were collected for analysis, and their original enrollment in Medicare could not be due to a disability or end-stage renal disease. A 1:1 matching of non-cancer controls with patients was performed based on age (year of birth) and race (white, black, Asian, Hispanic, Native American, other, and unknown). In cases where more than one non-cancer control subject matched a HER2- positive BC patient, the non-cancer control subject was randomly selected. We compared comorbidities and OS between HER2- positive BC patients and non-cancer controls.
Statistical analysis
Determining Diagnosis Date
The index date was defined as the first diagnosis date of HER2-positive BC. The full date of HER2-positive BC diagnosis (day/month/year) was required for the analysis. However, SEERMedicare only provided the year and month of diagnosis, so the diagnosis day was imputed. ‘Day’ was set to the 15th of every month, except February, when the diagnosis day was set to the 14th. If the diagnosis month was missing from the record, the month (and day) was imputed by searching the record for another diagnosis with a non-missing month, death, or last contact date in the same year and applying an algorithm to determine the diagnosis date. The same index date imputed for a HER2-positive BC patient was used as the index date for the matched non-cancer control.
Data Presentation and Survival Analysis
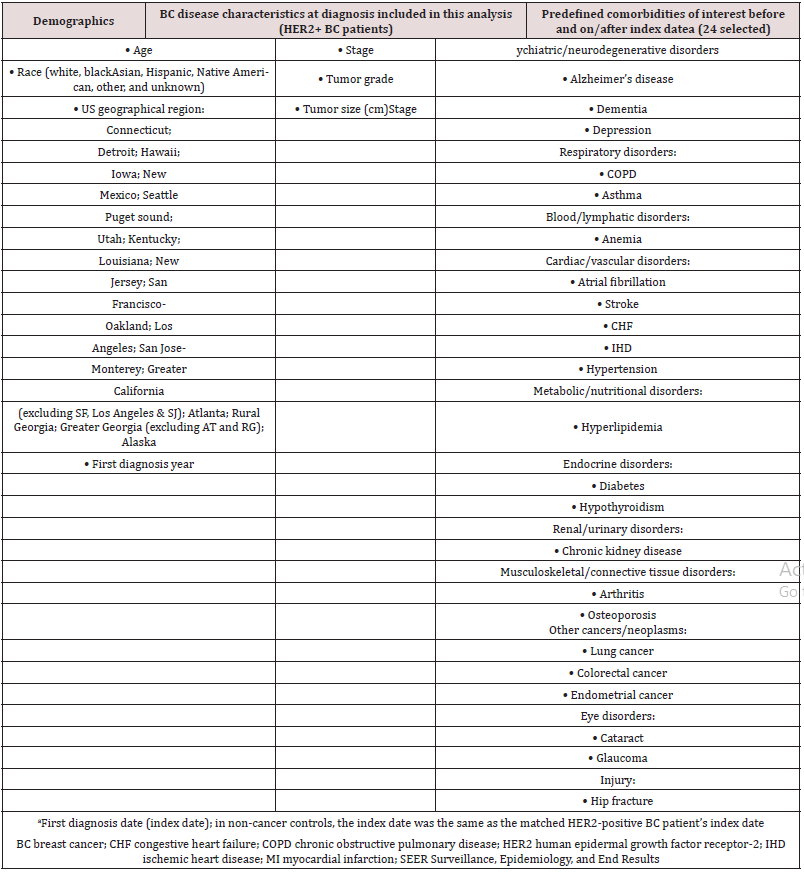
Summary statistics (mean, median, range, and quartile range) are presented for demographics, comorbidities, and deaths for both HER2-positive BC patients and non-cancer controls. Tumor stage, grade, and size information were also collected for HER2- positive BC patients. Cause of death (due to BC or any other cause) is reported for HER2-positive BC patients only. The prevalence of 24 predefined comorbidities (organized by system/organ class disorder) in HER2-positive BC versus non-cancer controls was assessed (Table 1). These 24 comorbidities represent a broad range of disease states that may be present in elderly (≥65 years of age) people. The Chi-square test was used to compare the frequency of each comorbid condition between HER2-positive BC cases and noncancer controls. Twenty-one of these comorbidities (excluding the three cancer comorbidities of endometrial cancer, colorectal cancer, and lung cancer) were also assessed ‘before’ and ‘on/after’ HER2- positive BC diagnosis (Table 1). Time to death due to HER2-positive BC was defined as the time from first diagnosis of HER2-positive BC to death due to BC. Patients were followed for a maximum of eight years after first diagnosis. Survival outcomes were estimated by the Kaplan-Meier (KM) method and compared using the logrank test for HER2-positive BC patients versus non-cancer controls. For those patients and control subjects who had not died at the time of data collection, the last known ‘alive’ date was recorded as December 31, 2017 for the purpose of this analysis. A univariate Cox proportional model for unadjusted data and a multivariate Cox proportional hazard model with an initial stepwise selection method for adjusted data were used to estimate the risk of dying for HER2-positive BC patients compared with matched non-cancer controls. Geographical US region and the 24 comorbidities (Table 1) were used in the selection process, with 0.1 as entry criterion and 0.05 as the stay-on criterion. Comorbidities prior to diagnosis of HER2-positive breast cancer that did not meet entry and stay-on criterion were removed from the multivariate model.
Table 1: Demographics, BC disease characteristics, and predefined comorbidities of interest obtained from SEER registries and used in the analysis.
Results
Patient population
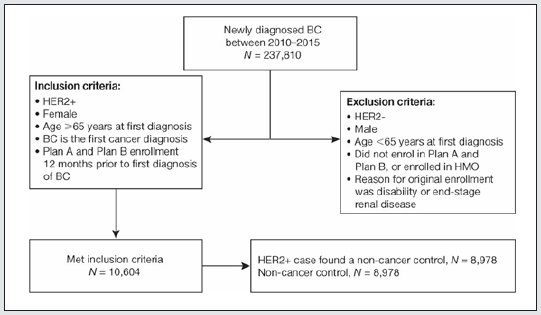
A total of 237,810 patients with newly diagnosed BC were registered and had data shared with the SEER-Medicare program between January 1, 2010 and December 31, 2015. Of these, 10,604 (4.5%) met the inclusion criteria for the current analysis. Of these 10,604 patients, we were able to identify age- and race-matched non-cancer controls in the 5% random sample of the Medicare population for 8,978 (84.7%) patients (Figure 1). Demographic information for HER2-positive BC patients and non-cancer controls is presented in Table 2. The mean age of HER2-positive BC patients and non-cancer controls was 77.1 years (range: 66.0-102.8 years) and the majority were white (82.0%). Just under 50% of HER2- positive BC patients and non-cancer controls were located in the West region of the US. Tumor stages across HER2-positive BC patients at diagnosis were: Stage 0, 6.2%; Stage I, 35.1%; Stage II, 33.2%; Stage III, 13.4%; and Stage IV, 8.4%. Just over half (50.9%) of patients had a Grade-3/poorly differentiated/high grade tumor, and the majority (91.7%) had a tumor size of 1-10 cm (Table 2).
Figure 1: Flow chart for screening newly diagnosed BC patients for potential inclusion in the SEER database analysis of elderly HER2-positive BC patients.
BC breast cancer; HER2-positive/HER2- human epidermal growth factor receptor-2. positive/negative; HMO Health Maintenance Organization; SEER Surveillance, Epidemiology, and End Results.
Comorbidities
Table 3 lists all 24 predefined comorbidities and their prevalence in HER2-positive BC and non-cancer controls. Thirteen comorbidities (13/24; 54.2%) were significantly more prevalent in HER2-positive BC patients compared with non-cancer controls overall (P < 0.05) and 3/24 (12.5%) comorbidities (P < 0.05) were significantly more prevalent in the non-cancer control group compared with HER2-positive BC patients (Table 3). Of the 13 comorbidities that were significantly more prevalent in HER2- positive BC patients, those with a higher overall prevalence (≥50%) in this group were: anemia (59.4% vs. 50.7%), hypertension, (72.1% vs. 69.5%), and arthritis (55.0% vs. 53.2%). The three comorbidities that were significantly more prevalent in noncancer controls compared with HER2-positive BC patients were Alzheimer’s disease, dementia, and hip fracture (Table 3). Of the 21 predefined comorbidities with ‘before’ and ‘on/after’ data (so excluding ‘other’ cancer comorbidities) in HER2-positive BC patients, there were significant differences in dementia, depression, asthma, COPD, anemia, CHF, IHD, chronic kidney disease osteoporosis, and arthritis between the two groups. Only hip fracture was more likely to be diagnosed ‘on/after’ the index date (51.1% [on/after] vs. 48.9% [before]), although the difference was small; the remaining 20 comorbidities were more likely to be diagnosed ‘before’ the index date (Table 3). Of those comorbidities that were diagnosed on or after the index date (although, overall, fewer than before diagnosis), all except two (hyperlipidemia and Alzheimer’s disease) were diagnosed at a higher rate in HER2- positive BC patients compared with non-cancer controls (Table 3).
Table 3: Comorbidities before and on/after HER2-positive BC diagnosis and between cases and non-cancer controls.
Survival Analysis
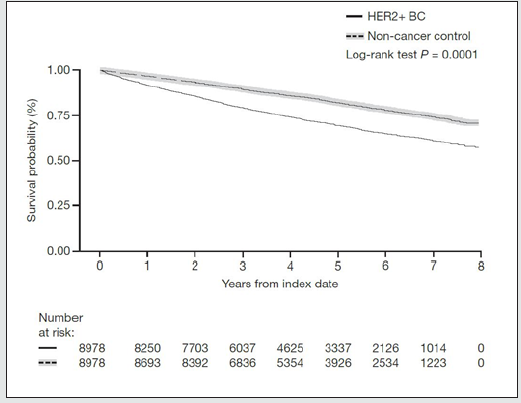
A total of 6246 (69.6%) HER2-positive BC patients and 7341 (81.8%) non-cancer controls were alive at data cut-off for the analysis (Table 4). Of the patients with HER2-positive BC who died during the study period, the cause of death was BC in 43.8% of patients; 48.0% died due to other disease and cause was ‘not available’ in 8.3% of patients (Table 4). As this component of the analysis did not adjust for comorbidities, it is likely that an important factor contributing to the deaths by ‘other disease’ is the presence of comorbidities. Figure 2 shows a KM plot of survival for HER2-positive BC vs. non-cancer controls. Using the log-rank test (unadjusted variables), time to death was significantly shorter for patients with HER2-positive BC versus non-cancer controls (P < 0.0001), and the unadjusted hazard ratio (HR) using the univariate Cox model was 1.84 (95% confidence interval [CI] 1.73-1.96) (Table 4; Figure 2). There were 3337 BC patients alive at 5 years versus 3926 non-cancer controls. The estimated probability of survival at 5 years for HER2-positive BC patients was 69.7% versus 82.4% for non-cancer controls. Comorbidities prior to diagnosis were used in the Cox regression model with step-wise selection of 0.1 as entry criterion and 0.05 as the stay-on criterion, 15 out of the 21 non-cancer comorbidities prior to diagnosis and region were selected for inclusion in the multivariate analysis (glaucoma, diabetes, depression, cataract, osteoporosis, arthritis and region were not selected as they did not meet the selection criterion). After adjusting for the 15 comorbidities, the risk of dying among HER2-positive BC patients was 1.934 times the risk of dying in noncancer controls (HR: 1.934 [95% CI 1.818, 2.057]; Table 4).
Figure 2: Kaplan-Meier survival curve of OS for HER2-positive BC patients and non-cancer controls after first diagnosis (index date).
The log-rank test was used to compare survival for HER2-positive BC cases versus matched non-cancer controls. BC breast cancer; HER2-positive human epidermal growth factor receptor-2 positive; OS overall survival.
Discussion
Published data describing the comorbidities and health outcomes of elderly (≥65 years) patients with HER2-positive BC are limited. Our retrospective cohort analysis using data from 18 registries associated with SEER-Medicare found that the prevalence of many comorbidities was significantly higher in HER2-positive BC patients compared with age- and race-matched non-cancer controls. For example, many of the comorbidities associated with cardiovascular disease (CVD) included in the list of 24 predefined comorbidities (congestive heart failure [CHF] and ischemic heart disease) and also risk factors for CVD (atrial fibrillation, hypertension, and diabetes) were significantly more prevalent in elderly patients with HER2-positive BC compared with non-cancer controls. Hyperlipidemia is also a CVD risk factor and showed a higher prevalence in HER2-positive BC patients compared with controls, but the difference was not statistically significant. Previous studies have shown that the presence of ≥2 CVD risk factors during or after BC treatment increases the risk of cardiac dysfunction [24]. For patients who develop cardiovascular comorbidities after BC diagnosis, an important reason may be adverse effects associated with adjuvant/neoadjuvant targeted therapy and/or chemotherapy [24]. Targeted therapies for HER2-positive BC can cause left ventricular cardiac dysfunction and CHF in some patients, and the severity of cardiac toxicity may increase with concomitant chemotherapy [7, 9, 10]. However, over half of the CVD-associated comorbidities and risk-factor comorbidities for CVD in our study were diagnosed before HER2-positive BC diagnosis, so it is unlikely that, for these patients, drug therapy for BC is contributing to the greater number of CV comorbidities or CVD risk factors versus noncancer controls.
In addition to CVD-related comorbidities and risk-factor comorbidities for CVD among HER2-positive BC patients in our study, anemia was also highly prevalent (≥50%) in HER2-positive BC patients relative to non-cancer controls (59.4% vs. 50.7%; P < 0.001). The proportion of patients diagnosed with anemia before BC diagnosis was 70.7%. The higher prevalence of pre-HER2- positive BC diagnosis anemia in our study is not surprising because anemia is relatively common in cancer patients [25]. Our HER2- positive BC cohort included 1955 (21.8%) patients with Stage III/IV BC at diagnosis, which increases the risk of anemia further if the bone marrow is impacted. Risk factors for anemia in cancer patients (outside of the risk imposed by chemotherapy/targeted treatments) are chronic diseases, iron deficiency, myelodysplastic syndromes, and bone marrow infiltration in cases of more advanced cancers [25-29] The presence of comorbidities such as those associated with CVD, a high number of CVD risk factors, and/ or anemia are associated with poor cancer outcomes and/or limit curative treatment options [7,9,10,24,30,31]. In BC patients who have comorbid CV risk factors and/or CVD at diagnosis, treatment options may be reduced if the risks of intervention (and associated cardiac toxicity risk) are likely to outweigh the benefits [7,9,10,24]. The presence of anemia before neoadjuvant therapy can result in patients being less likely to have a pathological complete response to treatment and inferior OS, and patients are also more likely to
progress to advanced cancer compared with cancer controls who do not have anemia [30,31]. In elderly patients with pre-existing CVD risk factors, comorbidities associated with CVD, or anemia, it is important that decisions on HER2-positive BC treatment should be made on an individual patient basis, with consideration of the number and severity of comorbidities as well as the appropriate management of these comorbidities.
However, it should be noted that although statistically significant differences in prevalence of comorbidities were found for 13 comorbidities that were more common in HER2+ BC patients and for three that were more common in non-cancer controls, many of these differences were small. For example, the difference in prevalence of hip fracture between HER2+ BC patients and noncancer controls was only about 1% (5.2% vs. 6.3%, respectively). Furthermore, the difference in prevalence of arthritis between HER2+ BC patents and non-cancer controls was less than 2% (55.0% vs. 53.2%, respectively). Though these differences were found to be statistically significant, their clinical relevance is questionable based on the results of this study, and this requires further research. Among patients with HER2-positive BC, 48.0% died due to a cause other than BC, suggesting that comorbidities may have been an important factor in these deaths. Included in these comorbidities are other cancers (lung, endometrial, and/ or colorectal cancer) that occurred after the BC diagnosis and showed a significantly higher prevalence in HER2-positive BC patients compared with non-cancer controls (3.7% vs. 0.7%) and may have also contributed to the mortality due to a cause other than BC. However, as cause-of-death data for these patients are not available, it is not possible to confirm this. In contrast, 43.8.% of HER2-positive BC patients had died as a result of BC. Time to death was significantly shorter for HER2-positive BC patients compared with non-cancer controls (4 years vs. 7 years; P < 0.0001 [log-rank test]) and the univariate Cox proportional model (unadjusted data) showed the risk of death was 1.84 greater for HER2-positive BC patients compared with non-cancer controls. To account for the potential impact of comorbidities on survival for HER2-positive BC patients versus non-cancer controls, a stepwise multivariate analysis was conducted. Using the stepwise approach, 15 out of the 21 predefined comorbidities prior to diagnosis were selected for multivariate analysis; there was still a 1.934 times greater risk of death in the HER2-positive BC cohort compared with subjects in the non-cancer control cohort. Given that this is based on data adjusted to account for comorbidity covariates, our data suggest that the patients with HER2-positiveBC in our study had a greater risk of death compared with non-cancer controls but that this may not be entirely associated with comorbidity. Another factor that may have contributed to the higher death rate in patients with HER2-positive BC relative to non-cancer controls after adjustment for comorbidities in our study is cancer stage. A high BC stage at diagnosis (and associated increased mortality risk relative to earlystage cancer) and the impact of BC progression through cancer stages over the ≤8-year follow-up period may have also influenced the results. Advanced BC stage (Stage III+) may be harder to treat (curative intent) than early-stage BC, particularly in elderly patients who have comorbidities, which may limit their treatment options [3].
In our study, a total of 1204 (13.4%) HER2-positive BC patients had Stage III and 751 (8.4%) patients had metastatic (Stage IV) disease at diagnosis – this represents 1955 (21.8%) of the total population at risk. In addition, 2977 (33.2%) patients had Stage II disease at diagnosis, and it is reasonable to hypothesize that some of these patients will have progressed to more advanced disease over the course of follow-up. However, it is important to note that cancer-stage data at diagnosis relative to cancer stage at the end of follow-up or death were not collected; having this information may have revealed a pattern between cancer stage and mortality risk. These assertions regarding cancer stage at diagnosis are supported by the results from a separate SEER analysis that included data from women aged ≥67 years with BC [32]. In this study, women with Stage I BC had improved comorbidity-adjusted survival compared with non-cancer controls, but survival was worse for women with Stage II or higher BC compared with non-cancer controls [32], hence supporting the suggestion that patients with Stage II or higher HER2-positive BC in our study were at higher risk of BC mortality than those with Stage I or 0 BC. The analysis also found that women aged ≥67 years with Stage I BC were more likely to die of CVD than of BC [32]. Although the number and severity of comorbidities present at BC diagnosis, along with age, race, and cancer stage, are important predictors of survival in BC patients, other prognostic factors such as socioeconomic status, recurrence, metastasis (BC not primary site) and secondary cancer, hormonereceptor status, and gene expression profile can impact on the longterm survival of patients with BC [33]. The comorbidity-adjusted HR for mortality of 1.84 relative to non-cancer controls in our analysis appears to support this assumption as there are factors other than comorbidities that are contributing to the mortality rate.
This analysis has a number of limitations. The analysis population identified from the SEER-Medicare program was predominantly white (82.0%), which is consistent with historic data showing the highest incidence of BC in non-Hispanic white women [34]; however, the limited number of patients from other racial groups means that the comorbidity and survival results of our study may not be an accurate reflection in other races and it is not possible to determine the effects of race on comorbidities or mortality. In addition, cancer-stage data for each patient at the end of follow-up or death (relative to cancer stage at diagnosis) were not collected. Having this data would have enabled us to determine how many patients had progressed through the different tumor stages over the 5-year period (2010-2015) of data collection and up to the end of follow-up (≤8 years). We did not collect data on how many comorbidities each patient experienced or the severity of these comorbidities – having these data would have enabled us to determine the overall comorbidity burden per patient compared with non-cancer controls. Including these additional analytic approaches may have further strengthened our findings. Despite the limitations, our study provides a valuable insight into the comorbidities and survival of elderly female patients with HER2- positive BC. As the study included data from almost 9000 patients in the SEER-Medicare program, it is reflective of real-world clinical practice and provides clinicians with information on the types of comorbidities likely to be encountered. It is important that clinicians consider the potential impact of comorbidities in the therapeutic management of patients with HER2-positive BC, and the need to effectively treat the comorbidities in the context of ongoing optimal cancer treatment.
Conclusion
Using data from the SEER-Medicare program, our study provides a valuable real-world insight into the comorbidities and survival of elderly female patients with HER2-positive BC. The higher incidence of comorbidities, including CVD-related comorbidities, in these patients compared to their age- and race-matched non-cancer controls highlights the importance of considering the impact of comorbidities in HER2-positive BC, particularly when considering treatment choices for individual patients. In addition, the higher mortality risk in HER2-positive BC patients compared with noncancer controls, both before and after adjusting for comorbidities, suggests additional factors are involved in survival.
Data Availability
The data which support the findings of this study are available from SEER-Medicare but restrictions apply to the availability of these data, which were used under a signed SEER Research Data agreement for the current study and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission from SEER.
Compliance with Ethical Standards
Conflict of interest NT, ZI, MH, and MS are employees of Daiichi Sankyo Inc. MDH is an employee of Rutgers University, NJ, USA, and is contracted to work at Daiichi Sankyo Inc. MS is also affiliated with the Center for Clinical Epidemiology and Biostatistics (CCEB)/ Center for Pharmacoepidemiology Research and Training (CPeRT) at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors, and the protocol required waiver from IRB.
Informed Consent:
As this study is based on a publicly available database without identifying patient information, informed consent was not required.
Acknowledgements
Medical writing support, under the direction of the authors, was provided by Lisa Moore, PhD, of CMC AFFINITY, McCann Health Medical Communications, funded by Daiichi Sankyo Inc., in accordance with Good Publication Practice (GPP3) guidelines.
References
- Williams GR, Mackenzie A, Magnuson A, Olin R, Chapman A, et al. (2016) Comorbidity in older adults with cancer. J Geriatr Oncol 7(4): 249-257.
- Patnaik JL, Byers T, Diguiseppi C, Denberg TD, Dabelea D (2011) The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst 103(14): 1101-1111.
- Tesarova P (2012) Breast cancer in the elderly-Should it be treated differently? Reports of practical oncology and radiotherapy. Rep Pract Oncol Radiother 18(1): 26-33.
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, et al. (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244(4905): 707-712.
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, et al. (235) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785): 177-182.
- Moasser MM (2007) The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 26(45): 6469-6487.
- (2009) Food and Drug Administration (FDA). Ado-trastuzumab emtansine (KADCYLA®) Prescribing information.
- von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, et al. (2019) Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med 380(7): 617-628.
- Food and Drug Administration (FDA). Trastuzumab (HERCEPTIN®) Prescribing information. 2010.
- Food and Drug Administration (FDA). Pertuzumab (PERJETA®) Prescribing information. 2012.
- Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, et al. (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13(1): 25-32.
- Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, et al. (2013) Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24(9): 2278-2284.
- Swain SM, Miles D, Kim SB, Im YH, Im SA, et al. (2020) Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol 21(4): 519-530.
- Food and Drug Administration (FDA). Fam-trastuzumab deruxtecan-nxki (ENHERTU®) Prescribing information. 2019.
- Modi S, Saura C, Yamashita T, Park YH, Kim SB, et al. (2020) Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med 382(7): 610-621.
- Food and Drug Administration (FDA) Neratinib (NERLYNX®) Prescribing information. 2017.
- Martin M, Holmes FA, Ejlertsen B, Delaloge S, Moy B, et al. (2017) Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 18(12): 1688-1700.
- (2019) Food and Drug Administration (FDA) Lapatinib (TYKERB®) Prescribing information.
- Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, et al. (2008) A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat 112(3): 533-543.
- Food and Drug Administration (FDA). Tucatinib (Tukysa®) Prescribing information 2020.
- Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, et al. (2020) Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N Engl J Med 382(7): 597-609.
- O'Sullivan CC, Suman VJ, Goetz MP (2019) The emerging role of CDK4/6i in HER2-positive breast cancer. Ther Adv Med Oncol 11: 1758835919887665.
- National Cancer Institute Surveillance Epidemiology and End Results Program. Derived HER2 Recode (2010).
- Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, et al. (2018) Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement from the American Heart Association. Circulation 137(8): e30-e66.
- Leonard RC, Untch M, Von Koch F (2005) Management of anaemia in patients with breast cancer: role of epoetin. Ann Oncol 16(5): 817-824.
- Busti F, Marchi G, Ugolini S, Castagna A, Girelli D (2018) Anemia and Iron Deficiency in Cancer Patients: Role of Iron Replacement Therapy. Pharmaceuticals 11(4).
- Ferrucci L, Balducci L (2008) Anemia of aging: the role of chronic inflammation and cancer. Semin Hematol 45(4): 242-249.
- Greenberg PL, Attar E, Bennett JM, Bloomfield CD, De Castro CM, Deeg HJ, et al. (2011) NCCN Clinical Practice Guidelines in Oncology: myelodysplastic syndromes. J Natl Compr Canc Netw 9(1): 30-56.
- Kopp HG, Krauss K, Fehm T, Staebler A, Zahm J, Vogel W, et al. (2011) Symptomatic bone marrow involvement in breast cancer--clinical presentation, treatment, and prognosis: a single institution review of 22 cases. Anticancer Res 31(11): 4025-4030.
- Zhang Y, Chen Y, Chen D, Jiang Y, Huang W, et al. (2014) Impact of preoperative anemia on relapse and survival in breast cancer patients. BMC cancer 14: 844.
- Zhu W, Xu B (2015) Association of Pretreatment Anemia with Pathological Response and Survival of Breast Cancer Patients Treated with Neoadjuvant Chemotherapy: A Population-Based Study. PloS one 10(8): e0136268.
- Schonberg MA, Marcantonio ER, Ngo L, Li D, Silliman RA, et al. (2011) Causes of death and relative survival of older women after a breast cancer diagnosis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 29(12): 1570-1587.
- Soerjomataram I, Louwman MW, Ribot JG, Roukema JA, Coebergh JW (2008) An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat 107(3): 309-330.
- American Cancer Society. Breast cancer facts and figures 2019-2020.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...