
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5945
Research Article(ISSN: 2638-5945) 
The Anti-Cancer Properties of Cannabidiol and Δ-9Tetrahydrocannabinol in Haematological Malignancies In Vitro Volume 4 - Issue 5
Bethan Hamill1, Saffran Pick1 and Lisa Lee-Jones1*
- 1Life Sciences Department, Manchester Metropolitan University, Manchester, United Kingdom
Received:August 19, 2021 Published: September 2, 2021
Corresponding author: Dr Lisa Lee-Jones, Department of Life Sciences, Manchester Metropolitan University, John Dalton Building, Chester Street, Manchester, M1 5GD, United Kingdom
DOI: 10.32474/OAJOM.2021.04.000200
Abstract
Background: Haematological malignancies are the fifth commonest cancer in the UK, with an average 5-year survival rate of 70.5%. Drug resistance and recurrence affect many patients with blood cancers. The anticancer properties of Cannabis constituents, Delta-9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD), in a variety of neoplastic cell lines, including those of the breast, skin, lung, and prostate, have been previously reported. This research investigated the cytostaticity and cytotoxicity of CBD and THC, synergism between CBD and THC, and chemosensitisation with Hydroxyurea (HU) in two haematological malignancy cell lines, Jurkat and U937.
Methods: U937 and Jurkat were incubated with various concentrations and combinations of CBD, THC, and HU for 24, 48, and 72 hours, then their effects on cell viability and proliferation were evaluated.
Results: Both CBD and THC reduced viability and proliferation in Jurkat and U937 in a time- and dose-dependent manner. CBD and THC demonstrated synergism in both cell lines. Combinations of cannabinoids and HU resulted in greater growth inhibition and cytotoxicity in both cell lines, than when any agent was used individually.
Conclusion: These findings suggest CBD and THC may be useful for treating haematological malignancies and warrant further in vitro studies.
Background
Haematological malignancies are the fifth most prevalent cancer in the UK, and its treatment is particularly challenging, often associated with severe toxicity [1]. Chemotherapy can result in the development of second primary malignancies [2]. Bone marrow transplantation offers curative potential [3]. However, multidrug resistance is a major cause of treatment failure and subsequent relapse in haematological neoplasms. The use of novel therapeutic agents as additional or adjuvant treatments for haematological malignancies may reduce recurrence, extend survival, and provide a cure without transplantation [3]. For thousands of years, people have acquired knowledge on the medicinal use of plants to treat a plethora of diseases. Indeed, many communities still rely on plantbased medicines, especially in developing countries [4]. The World Health Organisation has reported that traditional medicines are the only therapy available for 60% of the global population, either due to low income or distance from treatment centres [5]. Uganda is one such country that is reliant on several of its native 6,000 plant species, including Cannabis, to treat cancer [6, 7, 8]. Research into medicinal plants as novel therapeutic agents may represent a costeffective approach to drug discovery. 60% of clinically approved oncology drugs are derivatives of medicinal plants and therefore investigating the anti-cancer properties of medicinal plants may be an important means of identifying novel therapeutic agents [9]. Vinca alkaloids, a group of phytochemicals isolated from Catharanthus roseus, are used to treat numerous cancers including leukaemia, lung, breast, and liver. The four main vinca alkaloids clinically in use are vincristine, vindesine, vinorelbine, and vinblastine. Another plant-derived drug is Camptothecin, isolated from the bark and stem of Camptotheca acuminata, which inhibits topoisomerase I. Campothecin derivatives such as topotecan are used to treat ovarian and lung cancer [9]. Throughout history, Cannabis has been used for a multitude of purposes including food, recreational use, and religious rituals. Neolithic evidence from Taiwan, dated 12,000 years ago, suggests that Cannabis was used in cordage and manufacturing. The use of Cannabis for medicinal purposes dates back over 5,000 years [10]. However, the recreational use of Cannabis for its euphoric and psychoactive effects has restricted research into its clinical development [11]. Cannabinoids are naturally occurring compounds extracted from the Cannabis Sativa and Cannabis Indica plants. They are lipophilic ligands for cell-surface cannabinoid receptors (CB). Over 100 different cannabinoids have been described, with Delta- 9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD) receiving the most attention for their anti-cancer properties. CBD has antiinflammatory, neuroprotective and anti-psychotropic effects. THC reportedly induces analgesia, suppresses emesis, and stimulates appetite [12].
The human endogenous cannabinoid system has remained unchanged for over 500 million years. The system contains endocannabinoids, cannabinoid receptors, second messengers, and catabolic enzymes [13]. Cannabinoid receptors are located in cell membranes and are members of the G-protein-coupled receptor family. Cannabinoid receptor-1 (CB1) is highly expressed in the central nervous system, with moderate to low expression in the periphery. Cannabinoid receptor-2 (CB2) is highly distributed in the immune system, with restricted distribution in the central nervous system [14]. Cannabinoids bind to CB1 and CB2 receptors to activate pathways that result in apoptosis, autophagy and growth suppression [15]. CB1 and CB2 receptors are overexpressed in several cancer types, including breast, lung and prostate [12]. In non-small cell lung cancer, the expression of CB1 and CB2 receptors was 24% and 55% higher in vitro, respectively, when compared to normal lung tissue [16]. These findings suggested that CB1 and CB2 receptors could be used as novel therapeutic targets in non-small cell lung cancer [16].
Many studies have reported the antineoplastic effects of CBD and THC in cancer cell lines. CBD is non-psychoactive, unlike THC, and consequently has been more extensively investigated [17]. A previous study by McAllister et al. (2010) investigated the 72-hour (h) treatment of CBD [1.5μM] in the MDA-MB231 breast cancer cell line. They reported that CBD inhibited ID-1 expression and increased the production of reactive oxygen species. Furthermore, proliferation and invasiveness of the treated breast cancer cells were reduced by 90% and 70%, respectively, compared to the vehicle control [15]. Synergism of CBD and THC has been demonstrated in various neoplastic cell lines. A study conducted by Scott, Dalgleish and Liu (2017) investigated the effects of CBD and THC in the HL60 and CEM leukaemic cell lines. Both cannabinoids demonstrated cytotoxicity and growth inhibition when used as monotherapy. However, a greater effect was observed in both cell lines when they were used together. In the HL60 cell line, IC50 values were 12 μM, 16 μM, and 10 μM for cells incubated with CBD, THC and CBD plus THC (1:1), for 48h, respectively. The study concluded that synergism enabled a reduced dosage of both cannabinoids to be effective [18]. A reduced dosage is favourable to mitigate the psychoactive properties of THC, which currently limit THC’s therapeutic potential [19]. Both cannabinoids have also demonstrated the ability to chemosensitise cancer cells. Liu, et al. (2008) reported that THC chemosensitised leukaemia cell lines, (MOLT4, CEM, HL60), significantly reducing proliferation and viability. The IC50 value for CEM cells treated with cytarabine alone was 0.11 ± 0.014 μM, compared to 0.043 ± 0.0023 μM when cytarabine was used alongside THC [1 μM] [20]. Torres, et al. (2011) investigated a combination of THC and CBD in the U87MG and T98G glioma cell lines. They found that a combination of THC, CBD, and temozolomide (TMZ) strongly reduced viability, suppressed growth, and enhanced both autophagy and apoptosis. The combination of TMZ alongside both CBD and THC resulted in a greater decrease in viability of glioblastoma cells when compared to TMZ with either cannabinoid alone. In the U87MG cell line, cells treated with TMZ and THC displayed a 30% reduction in viability compared to the vehicle control. However, cells treated with TMZ, THC and CBD displayed a 60% reduction in viability, compared to the vehicle control [21]. Legislation changes pertaining to the medical and recreational use of Cannabis has permitted its evaluation in clinical settings. Clinical trials investigating cannabinoids for cancer have mainly focused on cancer-related symptoms, such as pain and cachexia [22,23]. There is extremely limited clinical trial data investigating Cannabis compounds for the treatment of malignancies. A phase I trial investigated intra-tumour administration of THC in nine patients with actively growing, recurrent glioblastoma. Patients undertook 1-6 cycles of treatment whereby single aliquots of THC solution were received via catheter into resection cavities. Median survival time was 24 weeks from the beginning of the cannabinoid treatment. THC induced apoptosis and decreased tumour cell proliferation [24]. A possible role of Cannabis in the regression of pilocytic astrocytoma in two paediatric cases has also been reported [25]. Both patients had previously undergone craniotomy and subtotal excision. MRI surveillance showed that one case displayed a slight increase in tumour size, while the other remained dormant. Tumour regression was observed in both cases over the next three years while the patients received Cannabis but no conventional adjuvant therapy, suggesting cannabinoid’s role in tumour regression [25]. A case study by Singh and Bali (2013) reported the dose-dependent management of a 14-year-old acute lymphoblastic leukaemia patient who had been unsuccessfully treated via traditional methods, (chemotherapy, radiation, bone marrow transplantation), using cannabinoid resin [26]. Despite the extensive research investigating the anti-cancer properties of THC and CBD, there is limited data investigating its efficacy in haematological neoplastic cell lines.
Methods
Reagents
20mM CBD (Tocris Bio-Techne, UK) stock was prepared in 100% ethanol. HU (200mM in ethanol) and THC (3.18mM in methanol) were purchased from SigmaAldrich, UK.
Cell Culture
The cell lines, Jurkat (T-cell lymphoblastic) and U937 (histiocytic lymphoma), were maintained in RPMI 1640 medium supplemented with 10% v/v foetal bovine serum, Penicillin (100 Units/ml), Streptomycin (100 Units/ml) and 2mM L-glutamax at 37°C in 5% CO2.
Proliferation and Viability Assay
To study the cytostaticity and cytotoxicity of CBD and THC, cells were seeded at 0.2E+06 cells/ml. 24h later, the investigative compound(s) or controls were added for 24, 48 or 72 h. The final concentrations evaluated were CBD [10 μM, 20 μM], THC [10 μM, 20 μM], and HU [200 μM] as monotherapy, or in combination. HU was used as the positive control. Vehicle negative controls were used. Ethanol was the vehicle for CBD and HU, methanol for THC, ethanol plus methanol were the vehicle for combinations of CBD plus THC, HU plus THC, and HU with CBD and THC. All conditions were set up in triplicate. Cell number and viability at all time points was determined using a trypan blue dye exclusion assay. The IC50 was determined using the GraphPad Prism 8 software, using additional concentrations of 5 and 15 μM CBD in U937. The cytotoxicity of CBD was compared against ethanol treated cells at each time point to identify the reduction in cell viability not attributable to the vehicle.
Statistical Analysis
All repeated measures were tested for normal distribution using IBM SPSS Statistics for Windows, version 25. One-Way ANOVA analysed the viable cell density for each condition evaluated, compared with the appropriate negative control, at each time point. If a significant difference was seen, a Post-hoc Dunnett’s test was used to identify which results were significantly different. Comparisons were also made between different concentrations of the cannabinoids, to determine whether increasing concentration had a significant effect. Viable cell density for combination treatment was compared against the respective single agents to investigate synergism and HU sensitisation. Viable cell density for all investigated conditions were also compared to the positive control at each time point, to determine their efficacy against an approved chemotherapeutic agent.
Data comparing responses between the cell lines was first tested for normal distribution, and an independent sample t-test was performed to identify significant differences for mean viable cell density in cells incubated with the same investigative compound(s), for the same period. P-value of <.05 was considered a significant difference.
Results
Growth Inhibition by CBD
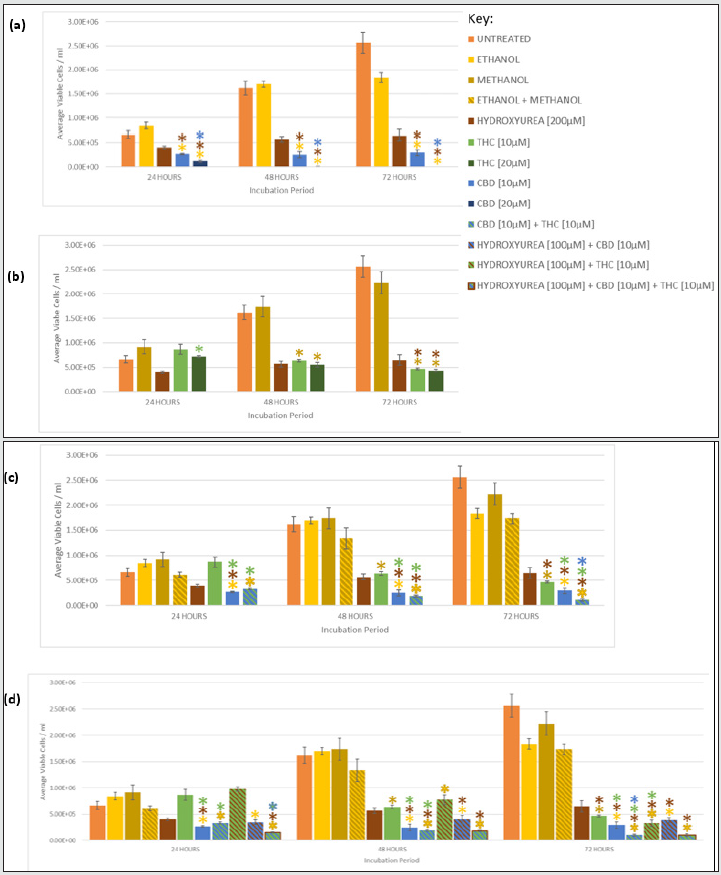
Both 10 and 20 μM CBD significantly reduced proliferation of Jurkat and U937 in a time- and dosage- dependent manner, compared to the vehicle, at all time-points (Figures 1a and 2a, Table 1). CBD [20 μM] was significantly better at suppressing growth than 10 μM CBD at all times in both cell lines (Table 1, Figure 1a). U937 cells incubated with CBD [10 μM] for 24, 48, and 72 h showed a 68%, 85% and 84% lower viable cell count, respectively, than the vehicle; whereas 20 μM further reduced it to 86%, 99%, and 100% lower than the vehicle after 24, 48, and 72 h, respectively (Figure 2a). Similar trends were observed in Jurkat (Figure 1a), however U937 demonstrated increased susceptibility to the cytostatic properties of CBD. Indeed, treatment with CBD [10 μM] in U937 for 24 and 48 h, caused significantly reduced proliferation compared to Jurkat (p= 0.044 and 0.005, respectively), which was also seen with 20 μM CBD for 24 and 48 h (p= 0.026, 0.033, respectively).
Growth Inhibition by THC
THC monotherapy also inhibited growth in a time- and dosagedependent manner (Figures 1b and 2b) however 10 μM THC only caused a significant reduction, compared to the vehicle, after 48 and 72 h, in both lines, whereas 20 μM THC did at all time-points in Jurkat, but again only after 48 and 72 h in U937 (Table 1). The higher concentration of THC, 20 μM, significantly suppressed growth compared to 10 μM THC at all time-points in Jurkat, but only after 24 hours in U937 (Table 1). The growth of Jurkat and U937 cells exposed to THC [10 μM] for 72 h was 73% and 79%, respectively, lower than the vehicle, respectively, and was further reduced to 82% and 81%, respectively, in 20 μM THC for 72 hr (Figure 1b, Figure 2b).
CBD was more cytostatic than THC
CBD [10 and 20 μM] inhibited proliferation more than THC [10 and 20 μM] in both cell lines, at all time-points. U937 treated with CBD [10 μM] demonstrated 38% less proliferation than when treated with THC [10 μM] after 72 h (Figure 2c). This reduction was increased to 100% when U937 was treated with 20 μM CBD compared with THC [20 μM] for 72 h (Figures 2a and 2b). A similar reduction was observed in Jurkat (Figure 1c).
Cytotoxicity of CBD
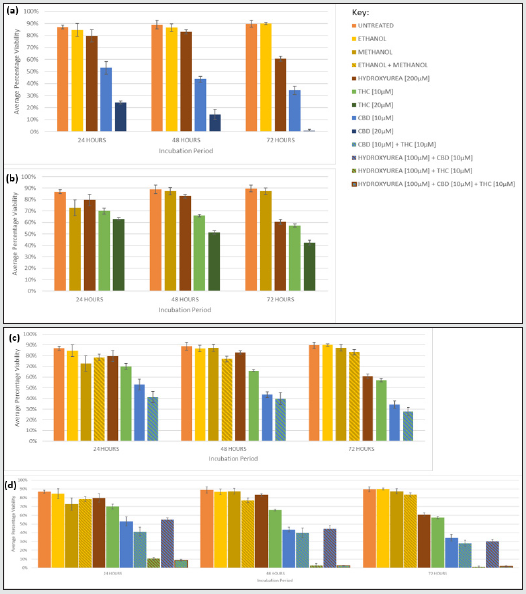
The cytotoxicity of CBD also followed a time- and concentrationdependent manner in Jurkat and U937 (Figures 3a and 4a). The greatest cytotoxicity was observed with 20 μM CBD resulting in only 1% and 0% viability in Jurkat and U937, respectively after 72 h.
Cytotoxicity of THC
THC caused cytotoxicity in a time- and dose- dependent manner in both cell lines (Figures 3b and 4b) and the reductions were similar. For example, the viability was reduced to 42%, and 46% when Jurkat and U937, respectively were exposed to THC [20 μM] for 72 h.
CBD was more cytotoxic than THC
CBD displayed greater cytotoxicity than THC at any concentration or time-point in both cell lines (Figures 3a, 3b, 4a, and 4b). CBD [20 μM] treated U937 cells were 100% less viable than THC [20 μM] treated cells after 72 h (Figures 4a and 4b). CBD, 20 μM, was the most cytotoxic monotherapy investigated at all time points, in both cell lines (Figures 3a and 4a).
Figure 1: Growth Inhibition of Jurkat by CBD and THC as monotherapies, synergism when CBD and THC were combined, and chemosensitisation of HU with THC and/or CBD. Graphs show mean viable cell density for Jurkat cells incubated with (a) CBD, (b) THC, (c) CBD and THC, and (d) CBD, THC and HU for 24, 48, and 72 hours. Mean viable cell count/ml were also included for untreated cells, vehicle controls (ethanol, methanol, ethanol and methanol), and the positive control HU. (*) indicates a statistically significant reduction (p <0.05) in cell density compared to the corresponding coloured investigative compound or control. Table 1 summarises the p-values for all comparisons.
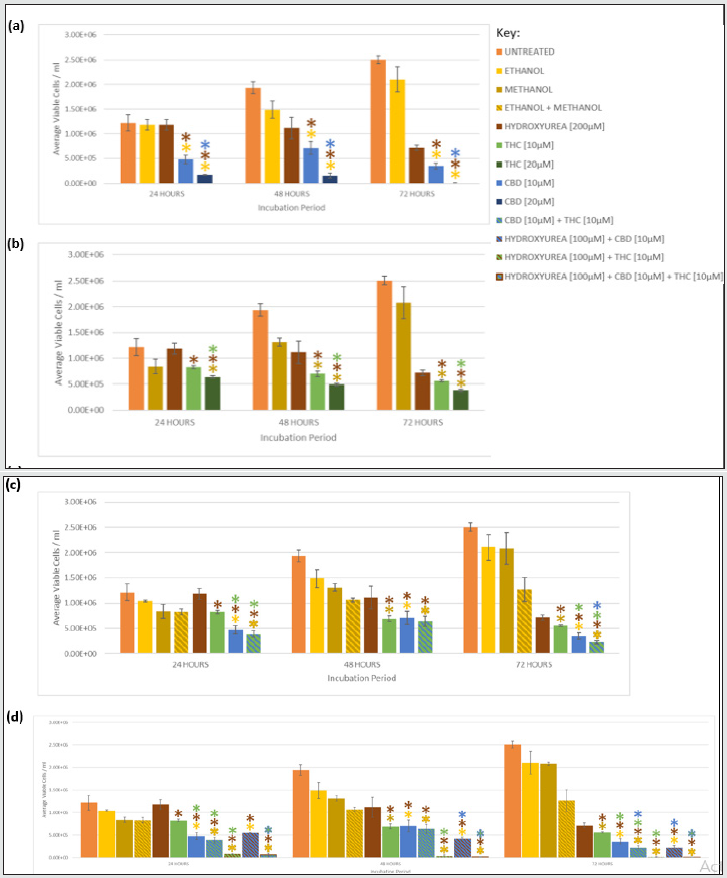
Figure 2: Growth Inhibition of U937 by CBD and THC as monotherapies, synergism when CBD and THC were combined, and chemosensitisation of HU with THC and/or CBD. Graphs show mean viable cell density for U937 cells incubated with (a) CBD, (b) THC, (c) CBD and THC, and (d) CBD, THC and HU for 24, 48, and 72 hours. Mean viable cell count/ml were also included for untreated cells, vehicle controls (ethanol, methanol, ethanol and methanol), and the positive control HU. (*) indicates a statistically significant reduction (p <0.05) in cell density compared to the corresponding coloured investigative compound or control. Table 1 summarises the p-values for all comparisons.
Synergism of CBD and THC
CBD [10 μM] plus THC [10 μM] resulted in greater growth suppression and cytotoxicity compared to either cannabinoid alone, at the same concentration, in both cell lines (Figure 1c, 2c, 3c, 4c), and the synergism was significantly greater in U937 compared to Jurkat cells after 48 and 72 h (p= .014 and .007, respectively). Only 12% of U937 incubated with CBD [10 μM] plus THC [10 μM] were viable after 72 h, this was 19% and 36% lower than compared to CBD [10 μM] and THC [10 μM] alone, respectively (Figure 4c). Meanwhile, only 28% of Jurkat cells treated with CBD [10 μM] plus THC [10 μM] were viable, which was 6% lower than CBD [10 μM] and 29% lower than THC [10 μM] monotherapies, after 72 h (Figure 3c).
Chemosensitisation by CBD and THC
A combination of cannabinoids and HU caused greater cytostaticity and cytotoxicity than when either of the compounds were used individually, indicating both cannabinoids sensitised cells to hydroxyurea, in both cell lines.
Chemosensitisation by CBD
Greater chemosensitivity, in terms of the cytostatic effects, to HU was observed in U937, with growth significantly reduced by 84% and 64% when incubated with HU [100 μM] plus CBD [10 μM] compared to when treated with HU [200 μM] alone, and CBD [10 μM] alone for 72 h, respectively (Figure 2d). However chemosensitivity was still apparent in Jurkat, with proliferation suppressed by 69% and 37% in the HU plus CBD treated cells compared to HU alone and CBD monotherapy, respectively, after 72 h (Figure 1d), however Jurkat had undergone significantly less proliferation compared to U937 cells for these conditions (p= .011).
Conversely, when evaluating cytotoxicity, chemosensitisation was less apparent in U937, with only a 14% reduction in viability for HU [100 μM] and CBD [10 μM] treated cells compared to HU [200 μM] monotherapy for 72 h (Figure 4d), whereas it was reduced by 30% and 3%, respectively, in Jurkat (Figure 3d).
Figure 3: Cytotoxicity of Jurkat cells exposed to CBD and THC as single agents, synergism when CBD and THC were combined, and chemosensitisation of HU with THC and/or CBD monotherapy. Graphs show the average percentage viability following 24, 48, and 72 hours of incubation with different concentrations of (a) CBD, (b) THC, (c) CBD and THC, (d) HU, CBD and THC, alongside untreated cells, vehicle controls (ethanol, methanol, ethanol and methanol), and positive control HU [200μM]. Error bars show mean ± s.d.
Chemosensitisation by THC
THC caused greater chemosensitisation to HU in Jurkat than in U937, and significantly reduced growth was evident at all timepoints in Jurkat compared to U937 (p= .000, .000, .011, for 24, 48, 72 h, respectively). Proliferation of Jurkat cells treated with HU [100 μM] plus THC [10 μM] for 72 h was 98% lower than when treated with HU [200 μM] or THC [10 μM] alone, for the same duration (Figure 1d); whereas it was only reduced by 50% and 30% compared to HU [200 μM] alone, and THC [10 μM] alone for the same duration in U937 (Figure 2d). Greater chemosensitisation to HU by THC was also apparent when cytotoxicity was evaluated in Jurkat compared to THC. Only 1% of Jurkat cells exposed to HU [100 μM] and THC [10 μM] for 72 h were viable, compared to 61% and 57% viability after HU [200 μM] or THC [10 μM], respectively, for the same period (Figure 3d). Meanwhile, 61% of U937 cells remained viable after 72 h with HU [100 μM] plus THC [10 μM], compared to 50% and 48% viability, respectively, in HU [200 μM] or THC [10 μM] (Figure 4d).
Figure 4: Cytotoxicity of U937 cells exposed to CBD and THC as single agents, synergism when CBD and THC were combined, and chemosensitisation of HU with THC and/or CBD. Graphs show the average percentage viability for Jurkat cells after 24, 48, and 72 hours with different concentrations of (a) CBD, (b) THC, (c) CBD and THC, (d) HU, with CBD and/or THC by CBD, alongside untreated cells, vehicle controls (ethanol, methanol, ethanol and methanol), and positive control HU [200μM]. Error bars show mean ± s.d.
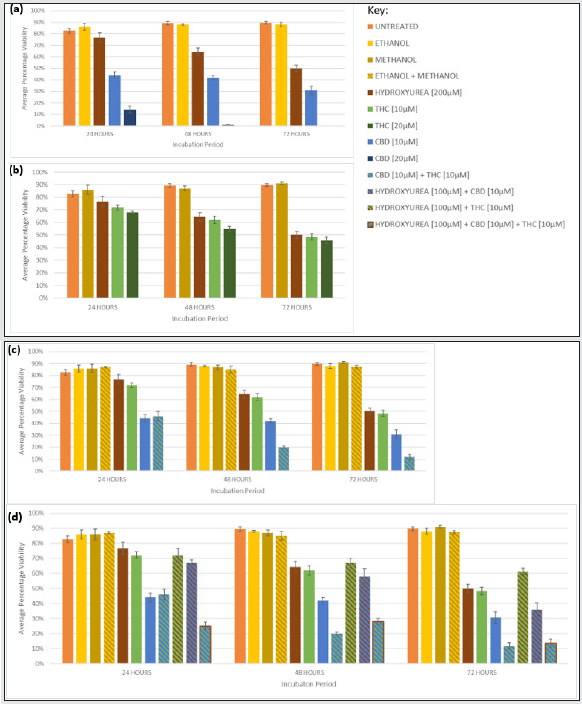
Chemosensitisation by CBD and THC
Greater chemosensitisation to HU in both cell lines was evident when CBD and THC were used combined, than when either agent was used individually and significantly less proliferation was observed in Jurkat than U937 for all time-points (p= .021, .000, and .024, for 24, 48, and 72 h, respectively, Figures 3d and 4d). A combination of HU [100 μM], CBD [10 μM] plus THC [10 μM] for 72 h in Jurkat reduced proliferation to 100% lower than HU [200 μM] alone, and 96% lower than CBD [10 μM] and THC [10 μM] (Figure 1d). Furthermore, only 2% of cells remained viable after Jurkat cells had been treated with HU [100 μM], CBD [10 μM] plus THC [10 μM] for 72 h, whereas 61% of cells treated with HU [200 μM] alone were viable, and 28% cells incubated with CBD [10 μM] and THC [10 μM] were viable (Figure 3d). Proliferation of U937 cells exposed to the triple combination for 72 h was 84% lower than when treated with HU [200 μM], and 2% less than CBD [10 μM] plus [THC 10 μM] for the same timeframe (Figure 2d). The viability of U937 incubated with all three agents for 72 h was 13%, whereas 50% and 12% viability were noted when incubated with HU [200 μM] alone or CBD [10 μM] plus THC [10 μM], respectively (Figure 4d).
Efficacy of CBD and THC Compared to HU
CBD [10 and 20 μM] monotherapy was significantly more effective at inhibiting growth than HU in Jurkat and U937, at all durations (Figures 1 and 2). THC [10 and 20 μM] was also significantly more efficacious than HU in Jurkat after 24, 48, and 72 hrs (Figure 1), but only in U937 after 72 h (Figure 2). CBD and THC dual therapy was also significantly more effective at suppressing proliferation than HU in Jurkat at all timepoints investigated (Figure 1), and in U937 after 48 and 72 h (Figure 2, Table 1). CBD and THC monotherapy and dual therapy resulted in a lower viability in both cell lines, at all-time points, compared to HU (Figures 3 and 4).
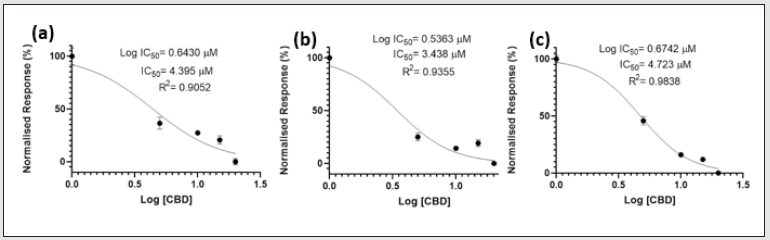
IC50 of CBD
IC50 values of CBD ranged from 3.438 – 4.723 μM (Figure 5). R2 values were close to 1 at all time-points (0.9052 – 0.9838), suggesting a strong relationship between viability and CBD concentration.
Figure 5: IC50 Values for U937 Cells Treated with CBD. U937 cells incubated with various CBD concentrations (5 to 20 μM) for (a) 24, (b) 48 and (c) 72 hours. IC50 values for 24, 48, and 72 hours were: 4.395, 3.438, and 4.723 μM, respectively. Data was analysed using the GraphPad Prism 8 software.
Discussion
CBD and THC were cytotoxic to U937 and Jurkat Cell Lines
Both CBD and THC significantly reduced the viability of the Jurkat and U937 cell lines, compared to their respective vehicle controls, in a dosage- and time-dependent manner. CBD at a concentration of 20 μM was the most cytotoxic monotherapy investigated and was more cytotoxic than THC at 10 and 20 μM, in both cell lines at each timepoint. No viable U937 cells remained after 72 h with CBD [20 μM], whereas 46% remained viable after THC [20 μM] for the same duration. These findings have been corroborated in other studies. For example, CBD demonstrated dose-dependent cytotoxicity in endometrial cancer cell lines, and reported greater cytotoxicity of CBD than THC in both cell lines after 72 h, at all concentrations evaluated (0.01 to 25 μM) [27]. CBD also demonstrated increased cytotoxicity with increased concentration in the breast cancer cell line, MDA-MB-231, with an increase in apoptosis from 40% to 90% when CBD increased from 5 μM to 10 μM. In addition, the percentage of apoptotic MDA-MB-231 cells increased from 18% to 41%, after 8 and 24 h of exposure, respectively, with 5 μM CBD [28].
Growth Suppression of Jurkat and U937 Cell Lines by CBD and THC
A significant growth reduction was evident in the Jurkat and U937 cell lines following treatment with CBD or THC compared to the vehicle control. These findings concur with Milian, et al. (2020) who investigated the cytostatic properties of CBD and THC in lung cancer cell lines, (A549, H460, H1792). Both CBD and THC reduced proliferation, compared to the vehicle control, in all cell lines tested
A concentration of 30 μM CBD and THC reduced proliferation in the A549 cell line by 40 and 20%, respectively, compared to the vehicle control after 48 h [29]. A time- and dose-dependent inhibition of proliferation by THC has also been reported in a malignant lymphoblastic cell line. EL4 cells were incubated with 1, 3, and 5 μM THC for 4, 8 and 12 h. The greatest growth inhibition was evident after 12 h in 5 μM THC which reduced growth by approximately 99%, whereas cells incubated with THC [1 μM] displayed no growth suppression, compared to the vehicle control [30].
Synergism of CBD and THC
The combination of CBD and THC resulted in greater growth suppression and cytotoxicity in both cell lines, compared to either cannabinoid alone at the same concentration, indicating synergism. These findings concur with those of Marcu et al. (2010) which demonstrated a combination of CBD and THC in glioblastoma cell lines, (U251 and SF126), acted synergistically to inhibit cell proliferation, cause modulations of the cell cycle, and induce apoptosis. SF126 cells incubated with CBD [1.1 μM] or THC [1.6 μM] for 24 h were 10 and 30% less viable, respectively, whereas cells incubated with both CBD [1.1 μM] and THC [1.6 μM] were 75% less viable, compared to the vehicle control. Specific changes such as modulations of extracellular signal-regulated kinase and caspase activities were only observed when the combination of cannabinoids was used, which may enhance the potential to inhibit a wider variety of malignancies [31].
CBD and THC Chemosensitise Jurkat and U937 to HU
This study demonstrated that combinations of one or both cannabinoids and HU resulted in increased cytotoxicity and growth inhibition in both cell lines. These findings agree with Torres, et al. (2011), who reported THC and CBD at a 1:1 ratio with TMZ greatly reduced the viability and proliferation, and enhanced autophagy and apoptosis in U87MG (CBD and THC [0.9 μM], TMZ [75 μM]) and T98G U87MG (CBD and THC [1.1 μM], TMZ [200 μM]) glioma cell lines. The combination of TMZ and both cannabinoids resulted in the greatest cytotoxicity of glioblastoma cells compared to TMZ, or either cannabinoid alone. In the U87MG cell line, 70% of cells treated with TMZ and THC were viable after 72 h, compared with only 40% of cells treated with TMZ, THC and CBD [21]. Jurkat cells displayed an increased susceptibility to the combination treatment when compared to U937. This difference in susceptibility could be due to different levels of CB1 and CB2 receptor expression between the two cell lines. The presence of CB2 receptors in Jurkat cells, and the stimulation of these receptors by THC, has been reported to cause pro-apoptotic and antiproliferative effects in vitro. A study conducted by McKallip, et al. (2002) found that Jurkat cells, expressing CB2 receptors, incubated with THC at 5 and 10 μM resulted in approximately 50% and 95% of cells undergoing apoptosis, respectively after 4 h [30]. There have been conflicting reports on the presence of CB receptors in U937 cells, with discrepancies seen considered to be due to different subclones of the cell line being used [32]. Liu, et al (2008) also reported that THC could sensitise cells to chemotherapeutic agents (cytarabine, doxorubicin and vincristine) in leukaemia cell lines, (MOLT4, CEM, HL60), significantly reducing proliferation and viability. The IC50 value for cytarabine alone in CEM cells was 0.11 ± 0.014 μM, compared to 0.043 ± 0.0023 μM when cytarabine was used alongside THC [1 μM] [20].
Nabissi, et al. (2012) found that CBD sensitised glioblastoma cells to temozolomide, carmustine and doxorubicin in vitro, due to CBD behaving as a selective transient receptor potential vanilloid type 2 (TRPV2) agonist. Increased apoptosis was evident when chemotherapeutic-treated glioma cells were incubated with CBD [10 μM] for three-days, compared to without [33].
Efficacy of CBD and THC Compared to HU
A significant growth reduction was evident in the Jurkat and U937 cell lines following treatment with CBD or THC compared to the positive control HU, an approved antiproliferative chemotherapeutic agent. There is limited data comparing the effect of cannabinoids versus chemotherapeutic agents alone. Literature focuses mainly on the efficacy of a combination of CBD and/or THC and chemotherapeutic agents versus chemotherapy alone. One study investigating the ability of CBD to sensitise glioblastoma cells (U87MG, MZC, NHA) to chemotherapeutic agents TMZ, carmustine, and doxorubicin found that CBD monotherapy was less effective than the chemotherapeutic agents alone. U87MG cells incubated with CBD, carmustine, or a combination of CBD and carmustine for 72 h, were approximately 90%, 65%, and 45% viable, respectively [33]. Torres et al. (2011) found that the antitumoral action of THC (15 mg/kg) and a combination of CBD (3.7 mg/kg) and THC (3.7 mg/kg) was less effective against human glioma cell lines (U87MG, A172, SW1783, U373MG, T98G, SW1088) than TMZ. The tumour weight of U87MG xenografts following 14 days of peritumoral injection with THC, CBD plus THC, and TMZ were 1.5g, 2.1g, and 1.0g, respectively (vehicle = 2.4g) [21].
IC50 of CBD
The IC50 values of CBD obtained in this study (3.438-4.723 μM) were similar to those reported by Scott, Dalgleish and Liu (2017) in the CEM human leukaemia cell line. Following 48 h of incubation with CBD, they found IC50 values for CEM cells were 7.8 ± 0.21μM. However, the IC50 value for CBD in the HL60 cell line was higher, 12μM, for the same period, presumably attributable to a wider range of concentrations being evaluated (1-50μM) or due to greater efficacy in this cell line [18].
Future Research
Investigating the antineoplastic efficacy of CBD and THC in additional haematological neoplastic cell lines is recommended, based not only because of these promising findings, but also as it is already established that immune cells have high levels of CB2 [14]. Furthermore, evaluating their cytotoxicity in non-neoplastic counterparts is also recommended. Further investigations addressing the efficacy of cannabinoids should be conducted on malignancies with elevated expression of CB1 and CB2. Investigations into the anti-tumourigenic potential of other cannabinoids, terpenes and flavonoids present in Cannabis are recommended [34], in addition to synthetic THC (dronabinol) and CBD (nabilone), which are approved to treat cytostatic-induced nausea and vomiting in cancer patients and to stimulate appetite in acquired immune deficiency patients [35]. The use of synthetic THC is particularly appealing as it lacks psychoactive properties.
Investigating sensitisation of additional chemotherapeutic agents by CBD and THC, alongside their scheduling is also recommended, as using cannabinoids after chemotherapy has resulted in greater induction of apoptosis [18]. Gene expression profiling and western blotting could be used to elucidate the pathways activated by Cannabis compounds, to facilitate determining the optimal combination of cannabinoids to use for the best treatment outcomes for different malignancies.
Conclusion
In summary, this research demonstrated that CBD and THC significantly reduced the viability and proliferation of Jurkat and U937 cell lines and sensitised them to the chemotherapeutic agent HU. Both cannabinoids acted in a time- and concentrationdependent manner in both cell lines. CBD [20 μM] was the most cytotoxic agent investigated as monotherapy, with Jurkat and U937 cell lines demonstrating 1 and 0% viability, respectively, after 72 h. Synergism between CBD and THC was identified at a 1:1 ratio (10 μM) when used together compared to either agent individually. A combination of CBD, and/or THC, with HU resulted in greater growth inhibition and cytotoxicity in both cell lines than HU monotherapy, suggesting cannabinoids can chemosensitise malignant cells to chemotherapeutic agents. Furthermore, CBD and/or THC was significantly more efficacious than HU in most conditions investigated. This research suggests cannabinoids have the potential to treat haematological malignancies, both as monotherapies or combined with each other and/or approved chemotherapeutic agents. Based on these findings, further investigation into the therapeutic use of cannabinoids for cancer is warranted.
Additional Information
Acknowledgements
The Life Sciences Department, MMU, is thanked for supporting this research.
Author Contributions
BKH designed and completed experiments, performed statistical analyses, and wrote the manuscript. SP assisted in experimental work. LLJ supervised research, provided laboratory training, guidance on experimental design and edited manuscript.
Ethics Approval
No ethical issues as the cell lines used in this research are commercially available.
Consent for Publication
Not applicable
Data Availability
Table 1 is available and can be uploaded in the online version if accepted.
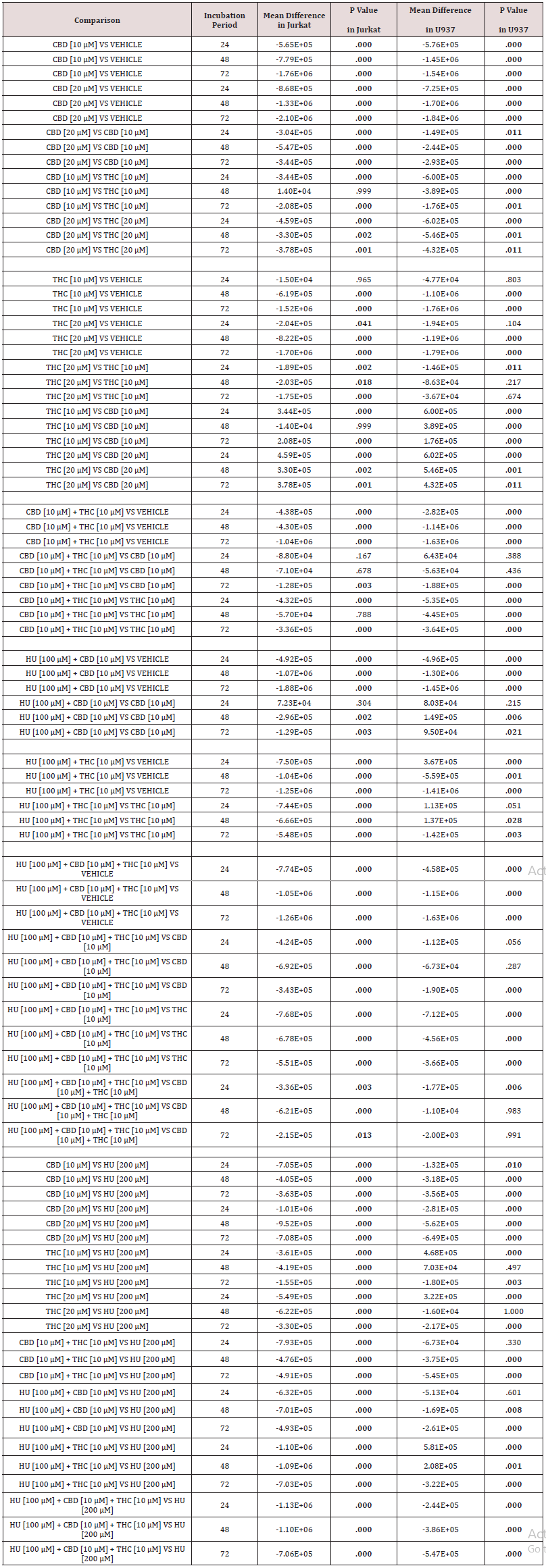
Table 1: Summary of p-values investigating growth inhibition of CBD, THC, and HU monotherapy or in various combinations in Jurkat and U937 cell lines. Table shows mean viable cell density following incubation with investigative compounds for 24, 48, or 72 hours, along with ±SD. Data was first tested for normal distribution, followed by one-Way ANOVA to analyse the viable cell density for each condition evaluated, compared with the appropriate negative control, at each time point. If a significant difference was seen, a Post-hoc Dunnett’s test was used to identify which results were significantly different. Comparisons were also made between different concentrations of the cannabinoids, to determine whether increasing concentration had a significant effect. Viable cell density for combination treatment was compared against the respective single agents to investigate synergism and HU sensitisation. Viable cell density for all investigated conditions were also compared to the positive control at each time point, to determine their efficacy against an approved chemotherapeutic agent. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 25.
Competing Interest
The authors declare no conflict of interest
Funding Information
The research consumables were provided by MMU. LLJ is an employee of MMU
Cell Line Authentication
The cells lines were purchased from the European Collection of Authenticated Cell Cultures.
References
- McCaughan D, Roman E, Smith AG, Garry A, Johnson M, et al. (2018) Determinants of hospital death in haematological cancers: findings from a qualitative study. BMJ Support Palliat Care 8(1): 78-86.
- Lenzi L, Lee-Jones L, Mostofa MA, Andrade DP, Ribeiro RC, et al. (2020) Second Primary Malignancy After Acute Promyelocytic Leukemia: a Population-Based Study. Cancers (Basel) 12(12): 3610.
- Kamareddine MH, Ghosn Y, Tawk A, Elia C, Alam W, Makdessi J, et al. (2019) Organic Nanoparticles as Drug Delivery Systems and Their Potential Role in the Treatment of Chronic Myeloid Leukemia. Technol Cancer 18: 1533033819879902.
- Pertwee RG (2006) Cannabinoid pharmacology: the first 66 years. Br J Pharmacol 147(1): S163-171.
- El-Seedi HR, Burman R, Mansour A, Turki Z, Boulos L, et al. (2013) The traditional medical uses and cytotoxic activities of sixty-one Egyptian plants: discovery of an active cardiac glycoside from Urginea maritima. J Ethnopharmacol 145(3): 746-757.
- Omara T, Kiprop AK, Ramkat RC, Cherutoi J, Kagoya S, et al. (2020) Medicinal Plants Used in Traditional Management of Cancer in Uganda: A Review of Ethnobotanical Surveys, Phytochemistry, and Anticancer Studies. Evid Based Complement Alternat Med 3529081.
- Arslan BA, Isik FB, Gur H, Ozen F, Catal T (2017) Apoptotic Effect of Nigella sativa on Human Lymphoma U937 Cells. Pharmacogn Mag 13(3): S628-s632.
- Alves-Silva JM, Romane A, Efferth T, Salgueiro L (2017) North African Medicinal Plants Traditionally Used in Cancer Therapy. Front Pharmacol p. 8: 383.
- Iqbal J, Abbasi B, Mahmood T, Kanwal S, Ali B, et al. (2017) Plant-derived anticancer agents: A green anticancer approach. Asian Pacific Journal of Tropical Biomedicine 7(12): 1129-1150.
- Bonini SA, Premoli M, Tambaro S, Kumar A, Maccarinelli G, et al. (2018) Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J Ethnopharmacol 227: 300-315.
- Maida V, Daeninck PJ (2016) A user's guide to cannabinoid therapies in oncology. Curr Oncol 23(6): 398-406.
- Sledzinski P, Zeyland J, Slomski R, Nowak A (2018) The current state and future perspectives of cannabinoids in cancer biology. Cancer Medicine 7(3): 765-775.
- Sharafi G, He H, Nikfarjam M (2019) Potential Use of Cannabinoids for the Treatment of Pancreatic Cancer. Journal of Pancreatic Cancer 5(1): 1-7.
- Rieder SA, Chauhan A, Singh U, Nagarkatti M, Nagarkatti P (2010) Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology 215(18): 598-605.
- McAllister SD, Murase R, Christian RT, Lau D, Zielinski AJ, et al. (2011) Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Research and Treatment 129(1): 37-47.
- Preet A, Qamri Z, Nasser MW, Prasad A, Shilo K, et al. (2011) Cannabinoid receptors, CB1 and CB2, as novel targets for inhibition of non-small cell lung cancer growth and metastasis. Cancer Prev Res (Phila) 4(1): 65-75.
- McAllister SD, Soroceanu L, Desprez PY (2015) The Antitumor Activity of Plant-Derived Non-Psychoactive Cannabinoids. Journal of Neuroimmune Pharmacology 10(2): 255-267.
- Scott KA, Dalgleish AG, Liu WM (2017) Anticancer effects of phytocannabinoids used with chemotherapy in leukaemia cells can be improved by altering the sequence of their administration. International Journal of Oncology 51(1): 369-377.
- Mersiades AJ, Tognela A, Haber PS, Stockler M, Lintzeris N, et al. (2018) Oral cannabinoid-rich THC/CBD Cannabis extract for secondary prevention of chemotherapy-induced nausea and vomiting: a study protocol for a pilot and definitive randomised double-blind placebo-controlled trial (CannabisCINV). BMJ Open 8(9): e020745.
- Liu WM, Scott KA, Shamash J, Joel S, Powles TB (2008) Enhancing the in vitro cytotoxic activity of Delta9-tetrahydrocannabinol in leukemic cells through a combinatorial approach. Leuk Lymphoma 49(9): 1800-1809.
- Torres S, Lorente M, Rodriguez-Fornes F, Hernandez-Tiedra S, Salazar M, et al. (2011) A Combined Preclinical Therapy of Cannabinoids and Temozolomide against Glioma. Molecular Cancer Therapeutics 10(1): 90-103.
- Inglet S (2021) Clinical Data for the Use of Cannabis-Based Treatments: A Comprehensive Review of the Literature. Ann Pharmacother 54(11): 1109-1143.
- Dumitru CA, Sandalcioglu IE, Karsak M (2018) Cannabinoids in Glioblastoma Therapy: New Applications for Old Drugs. Front Mol Neurosci PP. 11: 159.
- Guzmán M (2006) A pilot clinical study of Delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br J Cancer 95(2): 197-203.
- Foroughi M, Hendson G, Sargent MA, Steinbok P (2011) Spontaneous regression of septum pellucidum/forniceal pilocytic astrocytomas--possible role of Cannabis inhalation. Childs Nerv Syst 27(4): 671-679.
- Singh Y, Bali C (2013) Cannabis extract treatment for terminal acute lymphoblastic leukemia with a Philadelphia chromosome mutation. In: Case Rep Oncol 6(3): 585-592.
- Fonseca BM, Correia-da-Silva G, Teixeira NA (2018) Cannabinoid-induced cell death in endometrial cancer cells: involvement of TRPV1 receptors in apoptosis. Journal of Physiology and Biochemistry 74(2): 261-272.
- Shrivastava A, Kuzontkoski PM, Groopman JE, Prasad A (2011) Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol Cancer Ther 10(7) 1161-1172.
- Milian L, Mata M, Alcacer J, Oliver M, Sancho-Tello M, et al. (2020) Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. Plos One 15(2):e0228909.
- McKallip RJ, Lombard C, Fisher M, Martin BR, Ryu S, et al. (2002) Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood 100(2): 627-634.
- Marcu JP, Christian RT, Lau D, Zielinski AJ, Horowitz MP, et al. (2010) Cannabidiol enhances the inhibitory effects of delta9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol Cancer Ther 9(1) 180-189.
- Bari M (2006) Effect of lipid rafts on Cb2 receptor signaling and 2-arachidonoyl-glycerol metabolism in human immune cells. J Immunol 177(8): 4971-4980.
- Nabissi M, Morelli MB, Santoni M, Santoni G (2013) Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis 34(1): 48-57.
- Tomko AM, Whynot EG, Ellis LD, Dupré DJ (2020) Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers 12(7): (2020).
- Dariš B, Tancer Verboten, M, Knez Ž, Ferk P (2019) Cannabinoids in cancer treatment: Therapeutic potential and legislation. Bosn J Basic Med Sci 19(1): 14-23.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




