Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5945
Research Article(ISSN: 2638-5945) 
Radiofrequency Ablation for Atypical Cartilaginous Tumors is safe and effective: analysis of 189 consecutive cases Volume 3 - Issue 5
Nijland H1, Overbosch J2, Ploegmakers JJW1, Kwee TC2 and Jutte PC1
- 1Department of Orthopaedic Surgery, University Medical Center Groningen, The Netherlands
- 2Department of Radiology, University Medical Center Groningen, The Netherlands
Received: June 12, 2020 Published: June 29, 2020
Corresponding author: Nijland H, Department of Orthopaedic Surgery, University Medical Center Groningen, The Netherlands
DOI: 10.32474/OAJOM.2020.03.000172
Abstract
Objective: The purpose of this study was to investigate the efficacy and safety of radiofrequency ablation (RFA) as a less invasive treatment alternative for atypical cartilaginous tumors.
Materials and methods: Data of all consecutive RFA procedures for atypical cartilaginous tumors between 2007-2018 were analyzed, including temperature, amount of needle positions and ablation time per position. Tumor volume was measured on preoperative MRI and ablation zone was assessed on 3-month postprocedural MRI. RFA outcome parameters were ablation result (R0: complete with margin (≥2mm), R1: complete without margin (<2mm) and R2: incomplete) and occurrence of complications.
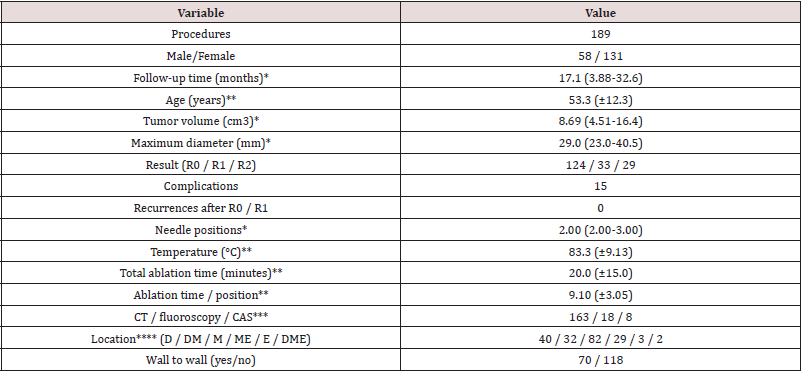
Results: In 84.4% of cases complete ablation was achieved (66.7% R0, 17.7% R1). In 15.6% of procedures ablation was incomplete (R2). No recurrences were seen after R0/1 ablations (with minimum two years follow-up). R0 was achieved significantly more frequent in smaller tumors (p = .027, odds ratio (OR) = 1.04 (per cm3) and with longer ablation time per needle (p = .048, OR = .894). Temperature >80°C (p = .026, OR = 7.57) resulted in more complete (R0 or R1) ablations without increasing complication rate (p = .579) compared to temperature of 71-80°C. In 15 procedures (7.9%) a complication occurred.
Conclusion: RFA provides promising results for treatment of atypical cartilaginous tumors with complete ablation (R0, R1) in 84.4% of procedures. Complication rates are comparable with open surgery and the amount of fractures is lower. These encouraging data support the potential of RFA to replace more invasive surgical approaches.
Introduction
Chondrosarcoma is the second most commonly diagnosed
primary bone tumor in adults (after osteosarcoma), with a
reported incidence of 8.78 per 1 million people [1-2]. They are
often found coincidently by MR, X-ray or CT imaging for unrelated
musculoskeletal symptoms [3]. Incidence increases proportionally
with the amount of imaging that is performed in the population
[4]. They can be divided into three grades based on histology
combined with macroscopic imaging features. Grade-1 central
chondrosarcomas in long bone are called atypical cartilaginous
tumors (ACTs) and are usually asymptomatic [3]. These tumors can
show locally aggressive growth, but metastases are very uncommon.
Prognosis of ACT is favorable, with 3-year overall survival of 96%
and 5-year overall survival of 93% [2].
Since ACTs are resistant to both radiotherapy and chemotherapy,
the first choice of treatment for these tumors has been surgical,
either by curettage or wide resection [5-6]. Recent literature
showed wide resection to be necessary only for tumors with local
aggressive growth [3]. A systematic review analyzing 214 curettage
procedures for ACT reported a success rate (i.e. no recurrent or
residual tumor) of 90.2%, with a complication rate of 4.8% (6 out of
126, of which five fractures) [5]. For wide resection complications
reportedly occurred in 24 out of 76 cases (= 31.6%) [5]. In a
large Cochrane analysis, a success rate of 93.2% was found for
curettage and 94.6% for wide resection [7]. In our center we found
comparable results in 108 patients, as described by Dierselhuis et
al. [3]. Recently, a wait- and see policy with radiological follow-up
has also been advocated [8].
With less invasive treatment functional outcome is better,
especially when limbs and joints can be preserved. Furthermore,
overall patient satisfaction is better, complication risk lower, weight
bearing is often not restricted, and hospitalization period is shorter
for minimally invasive procedures [9-10].
Radiofrequency ablation (RFA) has already been used in soft
tissue, primarily liver, for a long time. Application to bone tissue
has so far mainly been in treatment of osteoid osteoma [11-14].
In RFA tumor tissue is destroyed by local application of electricity,
causing motion, friction and heat and thereby causing coagulative
necrosis. The principle is based on the fact that (irreversible) tissue
damage starts at a temperature of 47°C and instantaneous protein
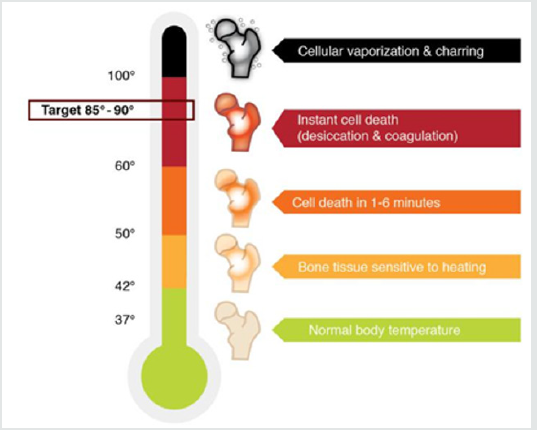
denaturation starts at 60°C (Figure 1).
Literature on RFA for treatment of bone malignancies is limited
and mainly focused on metastases [15-17]. Knowledge on safety
and efficacy of RFA treatment for ACTs, especially for larger tumors,
needs to be expounded. The aim of the current paper is to present
the data that were acquired over the last 12 years in the treatment of
ACT using RFA. Recommendations regarding safety and effectivity
of RFA for ACTs are provided.
(Figure1): Tissue reaction to heat. [18-20] Ideal temperature
for ablation is 60-95°C because instant cell death is the aim.
Above 100°C charring occurs, limiting conduction of heath and
thereby limiting the slow cooking effect of slow heat flow into the
surrounding [21].
Figure 1: Tissue reaction to heat [18-20] Ideal temperature for ablation is 60-95°C because instant cell death is the aim. Above 100 °C charring occurs, limiting conduction of heath and thereby limiting the slow cooking effect of slow heat flow into the surrounding [21].
Method
Procedure
Pre-RFA diagnosis of ACT was made based on imaging. On MR images they typically appear as a completely intramedullary located, popcorn-like lesion, with septonodular and peripheral enhancement after gadolinium-based contrast agent administration and limited scalloping. On T2-weighted imaging the chondroid components of an ACT appear as areas of high signal intensity. Perilesional edema and cortical breakthrough are signs of higher grade [22]. RFA was carried out in the department of radiology using CT guidance or in the operation room (OR) using fluoroscopy guidance or computer assisted surgery (CAS). This decision was dependent on tumor localization, size and comorbidities. Tumors with diameter over 6 cm were generally treated in the OR because of the possibility to place a prophylactic osteosynthesis to prevent fracture. Procedures were carried out under anesthesia. First a tract was drilled towards the tumor and a histological biopsy was performed to confirm the radiological diagnosis. Then an RFA needle was brought up towards the tumor and ablation was carried out. For the ablations from 2007-2014 a Boston Scientific RF3000 needle was used, from 2015-2019 a Cooltip needle (Medtronic). Parameters like ablation time per position and temperature were pre-operatively determined for every single procedure based on location in the bone, proximity to sensitive structures (cartilage, nerves, and vessels) and volume of the tumor. After the procedure these parameters were documented in the electronic patient file. Immobilization was only advised after procedures in the pelvic region. For all other procedures three months sports restriction (contact- and indoor sports) was advised.
At three months, baseline MR (consisting of unenhanced T1- weighted, fat-suppressed T2-weighted and gadolinium-enhanced sequences in two perpendicular planes with 4-mm slice thickness) was made to determine the exact ablation area. Subsequent followup MR scans were planned one, two, five and seven years after the procedure. For assessment of long-term effectivity only the data of the 85 patients with at 20 months follow-up was used.
Data Collection
Data, including histology results, from all consecutive RFA
procedures carried out in our center in a 12-year period (2007-
2018) were retrospectively collected from the prospectively kept
local bone tumor database and the patient files. In accordance
with regulations of the Medical Ethical Review Board of University
Medical Center Groningen (UMCG), patients were informed by
means of written information about the fact that anonymized data
of the procedure could be used for scientific research in case they
did not object. On pre-RFA MR images the tumor dimensions were
measured on T1- and T2-weighted scans. The observer was blinded
for final outcome during measurement. In three cases there was no
pre-RFA MR. these cases were excluded from the tumor volume
analysis. Treatment success (i.e. tumor completely within the RFA
halo on all MR sequences and slices) was determined on MR images
three months after RFA by a radiologist. Furthermore, the location
in the bone (diaphysis, metaphysis, and epiphysis) and whether the
tumor completely covered the distance between the medial and
lateral cortices (i.e wall to wall) was determined. From the tumor
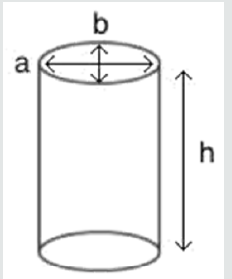
dimensions the volume was calculated using the following formula:
V = (p. r2) . H
V = volume
r = radius
h = height
Since most tumors were elliptical (see Figure 2) the formula
was split up:
V = (p . ½ . a . ½ . b) . h
a = width
b = depth
Which could be abbreviated to?
V = ¼. p. a . b . h
This formula describes the volume of an elliptical cylinder in
mm3; values were divided by 10^3 to be converted to cm3.
Data Analysis
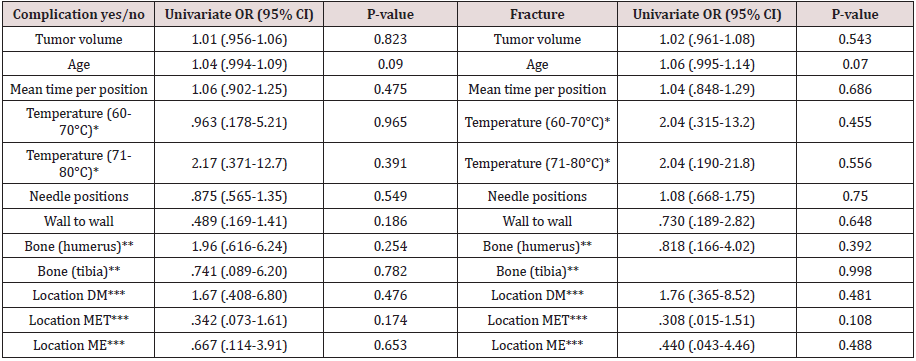
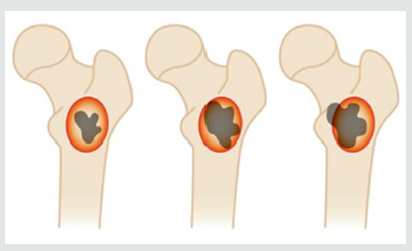
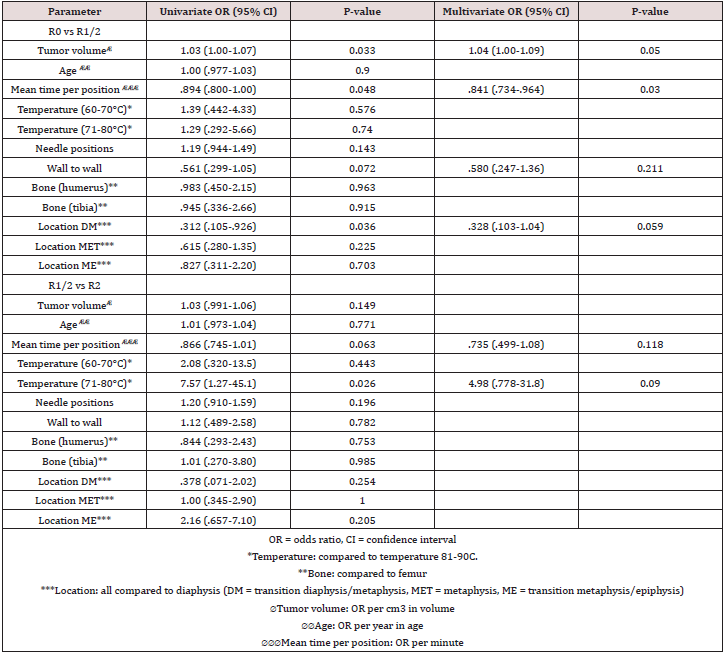
Outcome parameters were technical success and complications. We divided the technical success into three groups, according to Dierselhuis et al. [23]. Group 1 comprised complete ablations with a margin ≥2mm and was called R0, group 2 (R1) comprised complete ablations with a margin <2mm, and group 3 (R2) comprised of incomplete ablations (tumor visible outside the ablation zone or halo) (Figure 3). In our analysis we looked at the influence of peri-procedural parameters, including tumor volume, age, ablation time per position, temperature, amount of needle positions, wall to wall filling (i.e. from cortex to cortex), type of bone and location within the bone. Evaluation of tumor volume was preferred over maximum diameter because we think volume provides a better approximation of actual tumor size when determining if a tumor is suitable for RFA treatment. Maximum diameter possibly underestimates tumor size, especially for round tumors. In case of an R0 result, the ablation was considered technically successful, in case of R2 unsuccessful. R1 can be considered successful as well in case there is no local recurrence within two years (research with prolonged follow-up is ongoing). However, treatment aim was initially set at R0. Therefore, analysis was done for both R0 vs R1/2 and R0/R1 vs R2. We also analyzed the association between the peri-procedural parameters and a complication in general to occur, as well as between these parameters and a fracture (which was the most frequent complication). Applied temperature was divided into three groups (60-70, 71-80, 81-90°C ).
Figure 3: The 3 post-RFA result groups. R0 = complete ablation with ≥2mm margin, R1 = complete ablation with <2mm margin, R2 = incomplete ablation.
Statistical Analysis
Data were analyzed using SPSS Statistics v20 (IBM, Armonk,
United States). Data were tested on normality of distribution and
for correlation between variables. Analysis on the influence of periprocedural
variables on result and complications was done using
univariate logistic regression. Odds ratios, confidence intervals and
p-values were reported. For temperature we compared the 81-90°C
groups to the other two groups. Variables with p-value <.10 were
included in a multivariate logistic regression. Furthermore, the
odds ratios from logistic regression were used to look for a cut-off
point for tumor volume (to determine if there is a maximum tumor
volume from which the result is significantly worse). For all tests an
alpha of .05 was chosen.
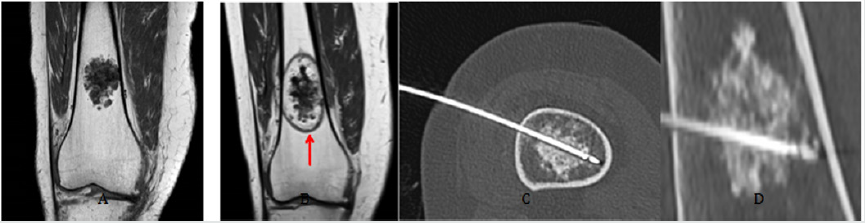
In figure 4 the RFA procedure is illustrated as well as images of
an ACT with and without needles in situ and a 3-month post-RFA
baseline T1-weighted MR.
Figure 4: Radiofrequency ablation of an atypical cartilaginous tumor. A: MR image of an ACT in distal femur.B: MR baseline, 3 months after RFA procedure. The red arrow indicates the ablation halo. C: Transverse oblique CT image with RF needle in situ. D: Sagittal oblique CT image with RF needle in situ.
Treatment result
In 124 procedures (=66.7%) R0 was achieved. In 33 procedures (=17.7%) the result was R1 and 30 procedures (15.6%) were R2. In six patients 3-month post-RFA baseline MR was not made due to debilitating amyotrophic lateral sclerosis (n=1), death from another disease (n=1), or complications (n=4: one avascular necrosis (AVN), two fractures (which necessitated surgical intervention with curettage and a plate) and one thermal skin injury (which necessitated surgical intervention)). The RFA result of these latter three cases was determined based on pathology at the time of surgical re-interventions. For the minimally invasive procedures 107 resulted in R0 (66.5%), 29 in R1 (18.0%) and 25 in R2 (15.5%). In four of the patients with R2 result a re-ablation was carried out. In three cases this led to complete ablation (three times R0), one patient still had a small residue. Considering the size (15x10x8mm) of this residue a wait and see policy was chosen. Follow-up showed no progression at 5 years after the procedure. (Table 2) depicts the effects of peri-procedural parameters between the three different treatment outcomes (R0, R1, R2). No correlations of >.50 were found between any variables. Based on univariate logistic regression for R0 vs R1/2, tumor volume (p = .033), time per position (p = .048), location on the transition of diaphysis to metaphysis (p = .036) and tumors reaching from wall to wall (p = .072) were included for multivariate analysis. In multivariate analysis only volume (p = .0450 and mean time per position (p = .013) had a significant effect. Larger tumor volume and shorter time per position lead to more incomplete (R1/2) ablations. The R2 of this multivariate model is .153, indicating only 15.3% of variance in result R0 vs R1/2 is due to the variables in the model. From the ablations with temperature between 60-70°C 68.4% resulted in R0, for 71-80°C this was 70.0% and for 81-90°C 75.0%. Only 5.4% of ablations over 80°C resulted in R2, compared to 17.2% for ablations up to 80°C. Based on univariate analysis for R0/1 vs R2 a temperature of 71- 80°C (compared to >80°C) (p=.026) and mean time per position (p = .063) were included for multivariate analysis. No variables had a significant effect on R0/1 vs R2. The multivariate model had an R2 of .171. For the 85 procedures (R0 or R1) with minimum 20 months follow-up no signs of residual tumor or recurrence were seen on MRI (median: 32.5 months, range: 20.0-115.4 months).
Table 2: Effect of most important peri-procedural parameters on treatment result (R0 vs R1/2 & R0/1 vsR2).
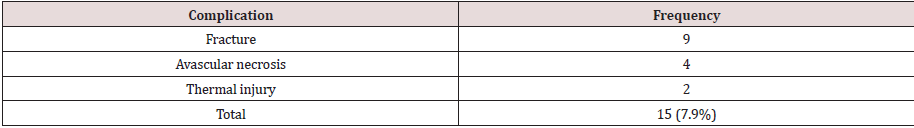
Complications
In 189 procedures a total of 15 complications (7.9%) occurred (table 3). The most frequent complication was a fracture (nine cases, 4.8%), followed by AVN (four cases, 2.1%). Seven out of nine fractures were found in the femur, the other two in the humerus. One case of thermal injury involved damage to the radial nerve, leading to loss of both sensory and motoric function. During four years of follow-up only partial motor function returned. The frequency distribution of the complications over the different bones is shown in table 5. Almost all fractures occurred in the femur and three out of four AVNs were in the humerus. Four out of nine fractures occurred on the transition diaphysis/metaphysis, three were in the diaphysis. All AVNs occurred in the proximal metaphysis/epiphysis.
Discussion
The current study represents a large series of 189 RFA procedures for ACT, of which 163 were carried out minimally invasive. No other studies on minimal invasive treatment of ACTs/ chondrosarcoma grade 1 have been published other than the smaller series of our group by Dierselhuis et al. [11, 23].
Treatment result
With regard to effective ablation our aim was to achieve
ablation with at least 2mm margin around the tumor. Therefore
the success rate of treatment could be calculated as R0/(R0 + R1 +
R2), which was 66.7% (124/186) in this study. This result is lower
than results for surgical treatment by curettage or wide resection
(90 to 95%) [3-5]. Nevertheless, a margin <2mm may be sufficient
as well, since the tumor still appears completely ablated. However,
a small margin might result in more local recurrences. Therefore,
a separate R1 group was created. When defining success to be a
complete ablation, the success rate could be calculated as (R0 + R1)
/ R2 = 157/186 = 84.4%, which is close to that of curettage of ACT.
For the CT-guided percutaneous procedures the success rate would
be 136/161 = 84.5%. To determine if R1 is a good result on the long
term, extensive follow-up period is required. In our series, none of
the R1 cases showed local residual disease activity on MRI during a
minimum follow-up period of two years.
RFA result was dependent on tumor volume. A larger volume
leads to a significantly higher risk of R1 or R2 ablation (p = .033).
For every cm3 increase in volume risk of R1/2 ablation increases by
4.4% (OR =1.044). Therefore, risk of an R1/2 ablation is double for
tumors over 16cm3 and threefold for tumors over 25cm3. However,
larger tumor volume does not significantly increase risk of an
incomplete ablation (R2) compared to a complete ablation (R0/1)
(p = .149). Therefore, larger tumors are also eligible for RFA.
However, given these findings more caution is advised in
deciding between minimal invasive- and open treatment for larger
tumors over 20cm3. Switching to open treatment however, leads
to a higher risk of complications, especially fractures (4-11% risk
of a fracture in curettage vs 10.5% in (wide) resection vs 4.8% in
minimally invasive RFA) [5, 25].
Our results show that ablations at lower temperature result
in more incomplete ablations (p = .026 for 71-80°C vs 81-90°C).
Ablations with 81-90°C result in 75% of cases in R0. Since all
ablations were carried out with temperature over 60°C, this finding
is contrary to the perception of instantaneous protein coagulation
to occur with temperatures over 60°C as presented in figure 1.
However, temperature measurement is done at the tip of the probe.
The ablation zone is larger than only the electrical zone as it is
formed by electrical conduction around the active zone of the needle
tip and the subsequent heat distribution with a certain gradient
within the surrounding tissue. With this distribution temperature
decreases at longer distance from the needle tip. Relatively low
ablation temperatures result in smaller ablation zones and more
chance of incomplete ablation. Since higher temperatures do
not significantly increase the risk of complications (p = .965 for
temperature >80°C compared to 60-70°C, p = .391 for temperature
>80°C compared to 71-80°C), safety is not compromised in high
temperature ablations (81-90°C). A point of consideration is that
for R0/1 vs R2, result is significantly different between 71-80°C
and 81-90°C, but not between 60-70°C and 81-90°C (p= .443). A
possible explanation is the fact that there were relatively a lot of
R1 ablations in the 60-70°C groups (21.1%). The fact that only for
R0/1 vs R2 a significant effect was found is due to the relatively
high amount of R1 ablations in the 81-90°C group (19.6%). Another
point of consideration is that temperature was only recorded in the
patient files in 88 procedures (=46.6%). With more data differences
might become more evident.
Shorter time per position leads to more R1/2 ablations (OR
= .894, p = .048), so longer ablation per position results in more
R0. This could be explained by the fact that temperature slowly
distributes through the bone during the ablation. Immediately after
finishing the ablation, tissue temperature starts to cool again. The
amount of needle positions does not affect the ablation result (p =
.143 for R0 vs R1/2, p = .196 for R0/1 vs R2) and the complication
risk is not dependent of increased time per position (p =.475).
Therefore, in the perspective of safe and effective ablation, it is
recommendable to perform ablations with longer time per position
instead of a large number of positions for large tumors. Experiments
are designed to confirm this hypothesis.
Ablations of ACTs with location on the transition from
diaphysis to metaphysis result in R0 significantly more often
compared to other locations. In 81.2% of cases R0 was reached,
compared to 57.5% in diaphysis, 68.8% in metaphysis and 62.1%
on the transition between metaphysis and epiphysis. There was no
difference in tumor volume, amount of needle positions, ablation
time per position or temperature between the different locations.
Complications
Temperature (p = .965 for temperature >80°C compared to 60-
70°C, p = .391 for temperature >80°C compared to 71-80°C), amount
of needle positions (p = .549) and tumor volume (p = .823) have no
significant effect on complication occurrence. Therefore, our data
do not indicate the complications were caused by overaggressive
treatment.
None of the peri-procedural variables affected the risk of a
fracture. The average age of the patients with a fracture was 60.8
years, compared to 53.0 years for patients without a fracture.
However, this difference was not significant (p = .070) and is most
likely due to the increasing incidence of fractures with age in the
population in general [24]. A possible solution could be to plan a
DEXA-scan before RFA-treatment in patients over 60 years old.,br>
The amount of complications after RFA of ACT (7.9%) was
similar to curettage surgery (5-11%) and low compared to
wide resection (31.6%) [5, 25]. To further reduce the risk of
complications our recommendation is to evaluate proximity to
structures particularly prone to heat damage (e.g. nerves, cartilage,
epiphyseal plate) carefully before the procedure. If the tumor is
not close to such a structure it may be safe to use more aggressive
ablation (longer time and higher temperature). Furthermore, since
a fracture (4.8% of cases) was the most frequent complication it
could be an option to introduce an immobilization period after
treatment, especially in femur, giving the bone better opportunity
to recover.
Points of consideration
A possible weakness of the current study is the fact that the parameter ablation time is hard to describe. Dependent on the shape and volume of the tumor, ablation is done using single- or multiple positions. In the current analysis ablation time is described as the time per position. Since positions are normally close to each other, an area is already (partially) preheated when ablation on the next position starts. Furthermore, the peri-procedural parameters evaluated in this study only account for a small portion of variance in treatment result (R2 = .153 for R0 vs R1/2 and R2 = .171 for R0/1 vs R2). In future evaluations more variables should be included to form a stronger model. Also, longer follow-up is necessary to be able to evaluate long-term effect of RFA treatment. A final point for consideration is the unpredictability of treatment result (i.e. the fact we encountered 17.7% R1 ablations). The aim is to be able to use this technique more accurately in the future as a result of increased experience and research, hereby guarantying complete ablation with the desired safety margin. To further expand the value of RFA for ACTs and to improve treatment result it would be interesting to use real-time feedback on halo size during the procedure. Option for this is MRI thermometry and dual energy CT [26-28]. However, so far these methods come with limitations like motion artefacts and unpredictability and need to be examined in further detail.
Conclusion
RFA provides promising results for the treatment of ACT with complete ablation (R0, R1) in 84.4% of procedures. Complication rate (7.9%) is comparable with open surgery. This is similar to results and complication rates as described in literature on surgical treatment [3-5]. There is less damage to surrounding tissue, costs are lower and it is more patient friendly because of the minimal invasive character. More research is needed to determine the optimal procedure parameters for ablation duration, needle positions and temperature. However, these encouraging data support the potential of RFA to replace more invasive surgical approaches for ACT treatment.
Contributors Statement
Literature search: H Nijland
Study design: H Nijland, PC Jutte
Data collection: J Overbosch, JJW Ploegmakers, TC Kwee, PC
Jutte
Data analysis/interpretation: H Nijland, TC Kwee, PC Jutte
Writing: H Nijland, J Overbosch, JJW Ploegmakers, TC Kwee, PC
Jutte
Disclosure statement
The authors declare that they have no relevant or material financial interests that relate to the research described in this paper.
References
- Primary bone cancer. [Internet]. US National cancer institute; 2019 Nov 20.
- Van Praag V, Rueten-Budde AJ, Ho V, Dijkstra PDS, Fiocco M, et al. (2018) Incidence, outcomes and prognostic factors during 25 years of treatment of chondrosarcomas. Surg Oncol 27(3): 402-408.
- Dierselhuis EF, Gerbers JG, Ploegmakers JJ, Stevens M, Suurmeijer AJ, et al. (2016) Local Treatment with Adjuvant Therapy for Central Atypical Cartilaginous Tumors in the Long Bones: Analysis of Outcome and Complications in One Hundred and Eight Patients with a Minimum Follow-up of Two Years. J Bone Joint Surg Am 98(4): 303-313.
- Stomp W, Reijnierse M, Kloppenburg M, de Mutsert R, Bovée JV, et al. (2015) Prevalence of cartilaginous tumours as an incidental finding on MRI of the knee. NEO study group. Eur Radiol 25(12): 3480-34877.
- Chen X, Yu LJ, Peng HM, Jiang C, Ye CH, et al. (2017) Is intralesional resection suitable for central grade
- 1 chondrosarcoma: A systematic review and updated meta-analysis. Eur J Surg Oncol. 2017 43(9):1718-1726.
- Campanacci DA, Scoccianti G, Franchi A, Roselli G, Beltrami G, et al. (2013) Surgical treatment of central grade 1 chondrosarcoma of the appendicular skeleton. J Orthop Traumatol 14(2): 101-107.
- Dierselhuis EF, Goulding KA, Stevens M, Jutte PC (2019) Intralesional treatment versus wide resection for central low-grade chondrosarcoma of the long bones. Cochrane Database Syst Rev 3(3): CD010778.
- Deckers C, Schreuder BH, Hannink G, de Rooy JW, van der Geest IC, et al. (2016) Radiologic follow-up of untreated enchondroma and atypical cartilaginous tumors in the long bones. J Surg Oncol114(8): 987-991.
- Reeves RA, DeWolf MC, Shaughnessy PJ, Ames JB, Henderson ER, et al. (2017) Use ofminimally invasive spine surgical instruments for the treatment of bone tumors. Expert Rev Med Devices 14(11): 881-890.
- Zou T, Li Q, Zhou X, Yang Z, Wang G, et al. (2015) Remove orthopedic fracture implant with minimal invasive surgery is good for the patient's early rehabilitation. Int J Clin Exp Med 8(12): 22377-22381.
- Dierselhuis EF, Overbosch J, Kwee TC, Suurmeijer AJH, Ploegmakers JJW, et al. (2019) Radiofrequency ablation in the treatment of atypical cartilaginous tumours in the long bones: lessons learned from our experience. Skeletal Radiol 48(6): 881-887.
- Callstrom MR, Charboneau JW ( 2005) Percutaneous Ablation: Safe, Effective Treatment of Bone Tumors. Oncology (Williston Park). 2005 11(Suppl 4): 22-26.
- Rosenthal DI, Alexander A, Rosenberg AE, Springfield D (1992) Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology 183(1): 29-33.
- Nijland H, Gerbers JG, Bulstra SK, Overbosch J, Stevens M, et al. (2017) Evaluation of accuracy and precision of CT-guidance in Radiofrequency Ablation for osteoid osteoma in 86 patients. Plos One 12(4):
- Nakatsuka A, Yamakado K, Uraki J, Takaki H, Yamanaka T, et al. (2016) Safety and Clinical Outcomes of Percutaneous Radiofrequency Ablation for Intermediate and Large Bone Tumors Using a Multiple-Electrode Switching System: A Phase II Clinical Study. J Vasc Interv Radiol 27(3):388-394.
- Tanigawa N, Arai Y, Yamakado K, Aramaki T, Inaba Y, et al. (2018) Phase I/II Study of Radiofrequency Ablation for Painful Bone Metastases: Japan Interventional Radiology in Oncology Study Group 0208. Cardiovasc Intervent Radiol 41(7): 1043-1048.
- Eriksson AR, Albrektsson T (1983) Temperature threshold levels for heat-induced bone tissue injury: A vital-microscopic study in the rabbit. J Prosthet Dent 50(1):101-107.
- Eriksson A, Albrektsson T, Grane B, McQueen D (1982) Thermal injury to bone. A vital microscopic description of heat effects. Int J Oral Surg 11(2):115-121.
- Feldman L, Fuchshuber P, Jones DB (2012) The SAGES Manual on the Fundamental Use of Surgical Energy (FUSE). Springer 15: pp. 266.
- Issa ZF, Miller JM, Zipes DP (1983)Ablation energy sources. Book: Advances in Radiation Biology
- Soldatos T, McCarthy EF, Attar S, Carrino JA, Fayad LM, et al. (2011) Imaging features of chondrosarcoma. J Comput Assist Tomogr 35(4): 504-511.
- Dierselhuis EF, van den Eerden PJ, Hoekstra HJ, Bulstra SK, Suurmeijer AJ, et al. (2014) Radiofrequency ablation in the treatment of cartilaginous lesions in the long bones: results of a pilot study. Bone Joint J 96-B(11): 1540-1545.
- Curtis EM, van der Velde R, Moon RJ, van den Bergh JP, Geusens P, et al. (2016) Epidemiology of Fractures in the United Kingdom 1988-2012: Variation with age, sex, geography, ethnicity and socioeconomic status. Bone 87: 19-26.
- Gerbers JG, Dierselhuis EF, Stevens M, Ploegmakers JJW, Bulstra SK, et al. (2018) Computer-assisted surgery compared to fluoroscopy in curettage of atypical cartilaginous tumors / chondrosarcoma grade 1 in the long bones. PLoS One 13(5): e0197033.
- Zhu M, Sun Z, Ng CK (2017) Image-guided thermal ablation with MR-based thermometry. Quant imaging med surg 7(3): 356-368.
- Zhou Y (2017) Noninvasive Thermometry in High-intensity focused ultrasound ablation. Ultrasound Q 33(4): 253-260.
- Paul J, Vogl TJ, Chacko A (2015) Dual energy computed tomography thermometry during hepatic microwave ablation in an ex-vivo porcine model. Phys Med 31(7):683-691.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...