Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5945
Review Article(ISSN: 2638-5945) 
Prognostic Value and Clinical Significance of Lncrna SNHG7 in Human Cancers: A Meta Analysis Volume 5 - Issue 1
Yulu Zhang1,2, Gang Liu1,3, Qing Deng1,3, Cong Li1,2 and Zhi Hua Li1,3*
- 1Department of Breast Surgery, Third Hospital of Nanchang, JiangXi Breast Specialist Hospital, Nanchang, People’s Republic of China
- 2Jiangxi Medical College of Nanchang University, Nanchang, People’s Republic of China
- 3Key Laboratory of Breast Diseases in Jiangxi Province, Nanchang, People’s Republic of China
Received: September 05, 2021 Published: September 24, 2021
Corresponding author: Zhi Hua Li, Department of breast, The Third Hospital of Nanchang City, Jiangxi Breast Specialist Hospital, Nanchang, Jiangxi 330009, China
DOI: 10.32474/OAJOM.2021.05.000201
Abstract
Background: SNHG7 is a newly identified lncRNA which can regulate tumour growth and metastasis.However, role of SNHG7 in the the
prognosis of various cancers is unclear.The aim of this meta analysis is to summarize the published studies to confirm the roles in tumor prognosis
and its clinical significance.
Methods: A literature search of online databases was performed in Pubmed,Web of science and Embase. Pooled HRs and the corresponding
95% CIs for OS ,as well as ORs and the corresponding 95% CIs for TNM stage, histological grade, tumor size, and lymph node metastasis were
evaluated.
Results: A total of seventeen studies with 1215 patients were enrolled in the meta analysis.Our results showed that high SNHG7 expression
level was associated with an unfavorable OS (HR=2.51, 95% CI: 2.08–3.04) for various tumors. In addition, it was found that increased SNHG7
expression was significantly associated with TNM stage (OR = 3.33; 95% CI:2.55–4.36), tumor size (OR = 2.16; 95% CI:1.61–2.9), lymph node
metastasis (OR = 3.84; 95% CI: 2.63–5.6). Nevertheless, there was no significant correlation between High SNHG7 expression level and histological
grade (OR=0.75;95% CI:0.51-1.09).
Discussions: High SNHG7 expression is predictive of poor OS, poor TNM stage, larger tumor size, and more lymph node metastasis, which
suggests High SNHG7 expression could serve as a independent novel biomarker of poor prognosis in cancer. But, additional studies are needed to
validate this observation.
Keywords: Lncrna SNHG7, Clinical Significance, Prognosis, Human Cancer
Introduction
Breast cancer is a common malignant tumor and the main cause of cancer death in women, ranked second only after lung cancer [1]. In 2018, there will be approximately 2.1 million newly diagnosed female breast cancer cases worldwide, accounting for almost a quarter of female cancer cases,and the cause of cancer death of breast cancer is about 11.6% [2]. LncRNAs have been demonstrated to play a key role in several aspects of carcinoma,comprises cell growth, differentiation, apoptosis and migration [3]. In recent years,small nucleolar RNA hostgene 7 (SNHG7),located on the chromosome 9[4], have Received a lot of attention.SNHG7 is associated with invasion and metastasis of various tumors, including breast cancer [5], cervical cancer [6], gastric cancer [4],prostate cancer [7] etc. However, whether SNHG7 can predict the prognosis of various cancers is unclear. In this meta, we summarize recent studies on the role of SNHG7 in cancers, to further analysis of the relationship with prognosis and clinical predictors.
Materials and Methods
Literature Retrieval Strategy
We comprehensively performed a search of EMBASE, PUBMED, WEB OF SCIENCE, from inception to April 2020. The search comprised the following terms:“breast cancer”, “carcinoma”, “SNHG7”, “tumor” and “tumorigenesis”.
Criteria for Inclusion and Exclusion
Eligible literature met the following criteria: (1) contains prognostic value no matter what kind of tumor; (2) provides the KM curve or hazard ratio (HR) of the overall survival rate and its 95% confidence interval; (3) provides odds ratio (OR) for clinic-pathological features; and (4) original research articles. Publications were excluded if they had one or more of the following criteria: (1) focus on animals instead of human; (2) abstract, review, comment, and case reports; (3) absence of survival outcomes, such as the hazard ratio (HR), 95% confidence interval (CI), and P values or OR values.and (4) insuffificient data.
Data Extraction and Quality Assessment
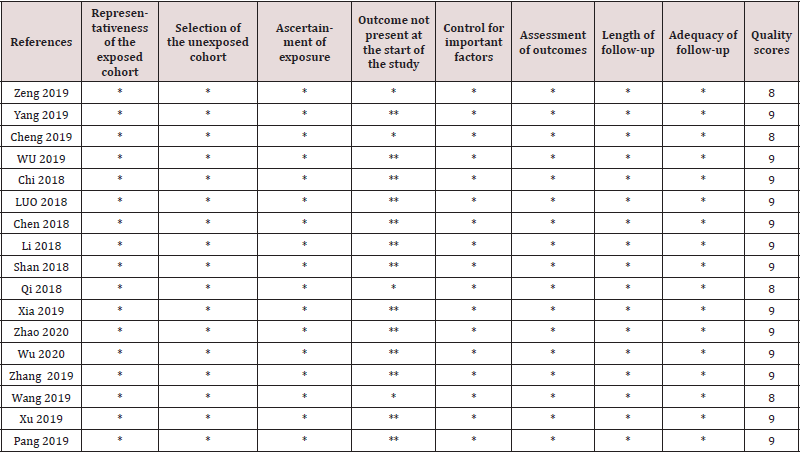
Data extraction and the assessment of quality were conducted independently by 2 investigators. A standardized table was used to record all available information,including (1).baseline information such as author,year,cut-off value,cancer type; (2).HR and its 95%CI; (3) .SNHG7 expression frequencies in different clinic-pathological features (TNM stage, histological grade, tumor size, and lymph node metastasis). For quality assessment of included studies, studies were respectively assessed by utilizing the modified Newcastle- Ottawa Scale (NOS). Any inconsistency was resolved by a senior investigator.
Statistical Analysis
The HR for OS was used as a measure of the relationship between SNHG7 expression and cancer prognosis. We utilized the hazard ratios(HRs) and 95% confidence intervals(CIs) reported in the original study when it was available ,and If not obtained directly from the article, the HR values were extracted from a survival curve through Engauge Digitizer (V.4.1) according the methodology mentioned by Tierney[8]. Odds ratios (ORs) and the corresponding 95% CIs were performed to assess the association between SNHG7 expression and clinic-pathological features, including TNM stage, histological grade, tumor size, and lymph node metastasis. Statistical analysis was performed with Review Manager 5.3 (The Cochrane collaboration, Oxford, UK).
Results
Characteristics of Eligible Studies
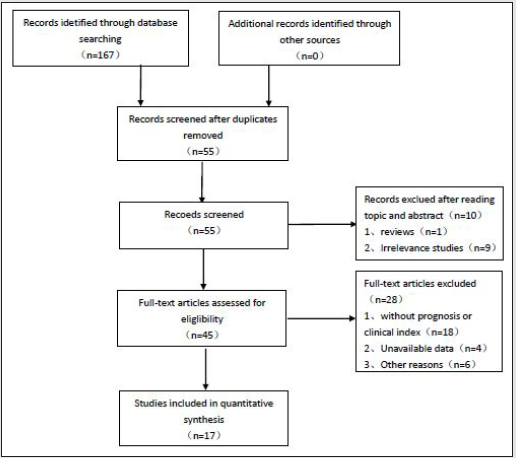
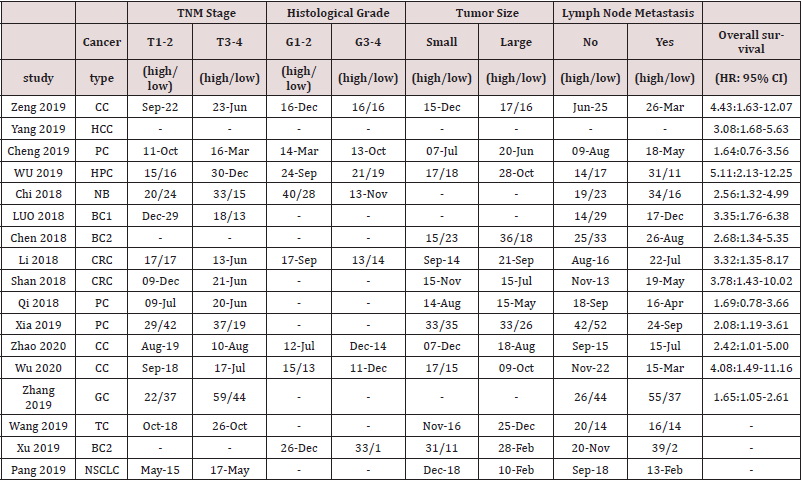
As shown in Figure 1,167 articles were initially obtained from Web of Science, PubMed, Embase.Of these, 112 studies were excluded because of duplication. After reading titles and abstracts,10 articles were removed as reviews or irrelevance theme. Then, 45 articles were reviewed in full,and 28 reports were excluded because they were articles lacking prognosis, clinical index,or availvable date. Eventually,17 studies with 1215 patients were included in the meta analysis. Main characteristics and data of articles included in this meta-analysis are described in table 1 and table 2. All articles are from china and were published between 2018 and 2020. The maximum and minimum sample size were 162 and 40, respectively. Of the enrolled studies, A total of 12 cancer types were included in our study: cervical cancer(n=3), hepatocellular carcinoma(n=1), Pancreatic cancer(n=1), hypopharyngeal cancer(n=1), Neuroblastoma(n=1), breast cancer(n=1), bladder cancer(n=2), colorectal cancer(n=2), prostate cancer(n=2),gastric cancer(n=1), Thyroid cancer(n=1),and non-small cell lung cancer(n=1).As the table 3 suggested,according to the modified NOS,All inclued studies got scores of 8 or more, indicating the high methodological quality.
Table 1: Main characteristics of articles included in this meta-analysis CC=cervical cancer, HCC=hepatocellular carcinoma, PC1=Pancreatic cancer, HPC=hypopharyngeal cancer, NB=Neuroblastoma, BC1=breast cancer, BC2=bladder cancer, CRC=colorectal cancer, PC2=prostate cancer, GC=gastric cancer, TC=Thyroid cancer, NSCLC=non-small cell lung cancer, OS=overall survival.
Table 2: Main data from articles for this meta-analysis.
Tissue SNHG7 Level and Overall Survival
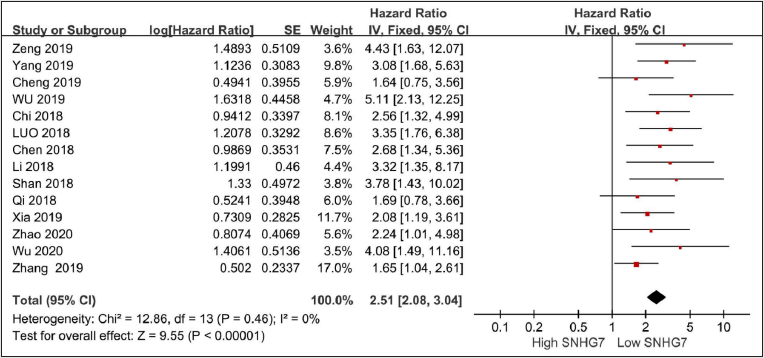
Fourteen studies with 1037 patients reported on overall survival[9-22]. There is no heterogeneity among these studies (I2 = 0%, P=0.46). Under the fixed effects model, the HR for OS was 2.51(95%CI=2.08-3.04,P<0.00001), indicating that high SNHG7 predicted poor OS for cancer patients.
Tissue SNHG7 Level and Clinic-Pathological Features
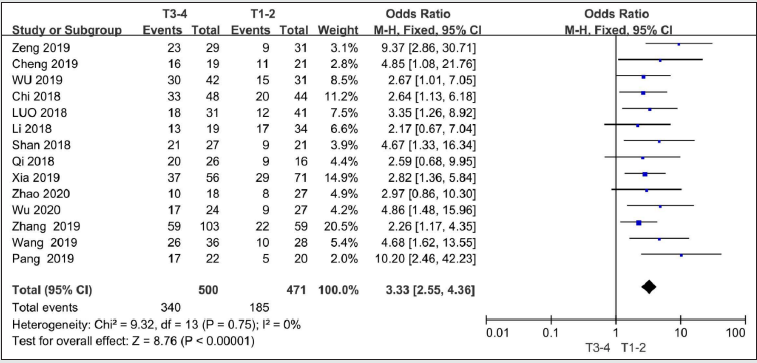
Fourteen studies with 971 patients reported on TNM stage [9, 11-14, 16-24]. There is no heterogeneity among these studies (I2 = 0%, P=0.75). Under the fixed effects model,the OR for TNM stage was 3.33(95%CI=2.55-4.63,P<0.00001), indicating that high SNHG7 predicted poor TNM stage for cancer patients.
Figure 3: Forest plot of ORs for TNM stage of high SNHG7 expression vs low expression in cancer patients.
Tissue SNHG7 level and Histological Grade
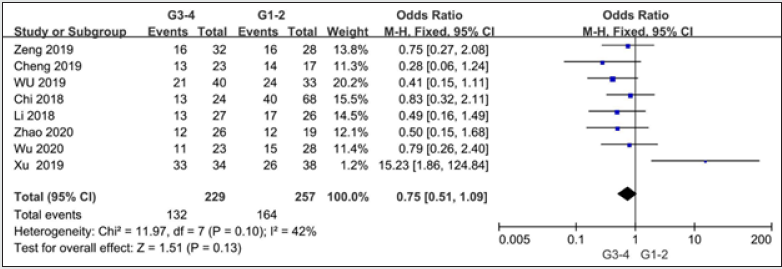
Eight studies with 486 patients reported on histological grade [9, 11-13, 16, 20, 21, 25]. There is no heterogeneity among these studies (I2 = 42%, P=0.1). Under the fixed effects model,the OR for TNM stage was 0.75(95%CI=0.51-1.09,P=0.13), indicating that SNHG7 level had no relation with histological grade.
Figure 4: Forest plot of ORs for Histological grade of high SNHG7 expression vs low expression in cancer patients.
Tissue SNHG7 level and Tumor Size
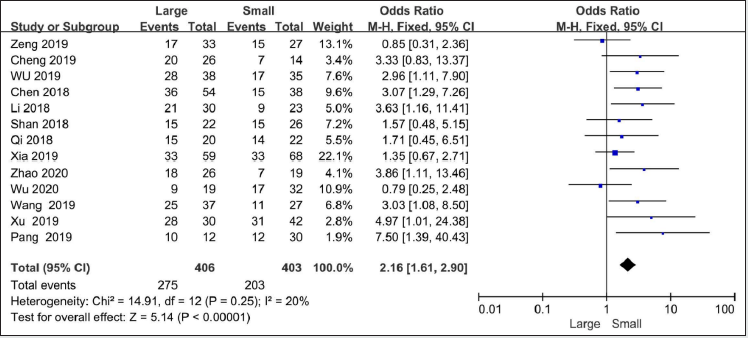
Thirteen studies with 809 patients reported on tumor size[9, 11, 12, 15-21, 23-25]. There is no heterogeneity among these studies (I2 = 20%, P=0.25). Under the fixed effects model,the OR for tumor size was 2.16(95%CI=1.61-2.9,P<0.00001), indicating that high SNHG7 predicted larger tumor size for cancer patients.
Figure 5: Forest plot of ORs for Tumor size of high SNHG7 expression vs low expression in cancer patients.
Tissue SNHG7 Level and Lymph Node Metastasis
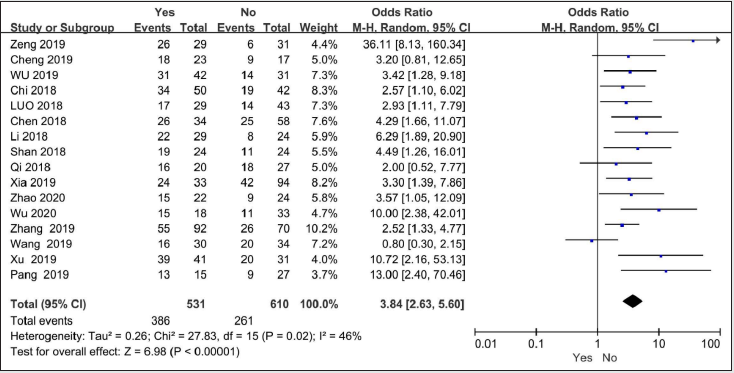
sixteen studies with 1135 patients reported on lymph node metastasis[9, 11-25]. There is moderate heterogeneity among these studies (I2 = 46%, P=0.02). Under the random effects model,the OR for lymph node metastasis was 3.84(95%CI=2.63-5.6,P<0.00001), indicating that high SNHG7 predicted more lymph node metastasis for cancer patients.
Figure 6: Forest plot of ORs for Lymph node metastasis of high SNHG7 expression vs low expression in cancer patients.
Discussion
As the incidence of cancer increases, more cancer patients
suffer physical and mental damage [1]. Metastasis is the main cause
of death of cancer patients, but it’s exact mechanism is still unclear.
Recently, researchers have focused on new prognostic molecules
markers in order to find specific metastasis mechanisms to enable
patients to receive more accurate treatment. As a newly discovered
molecule related to tumor prognosis, Chaudhry [26] first reported
in 2013 that SNHG7 is a small nucleolar RNA host gene, which is
expressed in lymphoblastic cell lines TK6 and wtk1.
Later research has shown that SNHG7 is a key oncogene in
many human cancer types, including breast cancer, cervical cancer,
hepatocellular carcinoma, Pancreatic cancer, hypopharyngeal
cancer, Neuroblastoma, bladder cancer, colorectal cancer, prostate
cancer, gastric cancer, Thyroid cancer, non-small cell lung cancer [9-
25, 27]. Rencently, more and more studies reportd the prognostic
value of SNHG7 overexpression for different cancers. Zhang, et al
[28] reported that High levels lncRNA SNHG7 indicated shorter
recurrence and OS time Synchronous Colorectal Liver Metastasis
Following Hepatectomy She K, et al [29]. demonstrated that
SNHG7 can stimulate the proliferation, migration and invasion
of lung cancer cells and suppress their apoptosis by improving
FAIM2 expression Guo, et al [30]. suggested that SNHG7 promoted
proliferation and migration by sponging miR‐449a in thyroid
cancer cells. And other studies demonstrated that SNHG7 accelerate
proliferation and tumorigenesis interacting with miR-34a through
EMT initiation and the Notch-1 pathway in breast cancer [5,31],
through PI3K/Akt/mTOR pathway in colorectal cancer[16],through
EMT in osteosarcoma[32], and through EMT in gastric cancer [22].
However, Only one other study hold that SNHG7 curbed malignant
transformation in uveal melanoma cells [33]. These studies found
that lncRNA SNHG7 may serve as an key prognostic factor in various
human rumors. Until now, the underlying regulatory mechanisms
by which SNHG7 regulated human cancers and its utility as a
biomarker remained unclear. In our meta-analysis, we explored
prognostic value and clinical significance of LncRNA SNHG7 in
human cancers.
A total of 1215 patients with various cancer from the 17 eligible
studies were systematically collected and assessed in this study.
High SNHG7 expression is predictive of poor OS, poor TNM stage,
larger tumor size, and more lymph node metastasis. Despite our
pooled analysis showes a comprehensive study, this meta-analysis
has some limitations. Firstly, this study include the small sample
size. Secondly, The HR values of only two studies are obtained
directly from the text. Thirdly, all included articles are from China.
Finally, most of the included articles are retrospective cohort
studies. In summary, We suggests that high SNHG7 expression
could serve as a independent novel biomarker of poor prognosis
in cancer. Additional multicenter prospective studies are required
to confirm our results and a more precise molecular mechanism.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Contract grant numbers: 81860546).
Disclosure Statement
The authors declare no conflict of interests.
References
- Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer. J Clin 70(1): 7-30.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6): 394-424.
- Su M, Xiao Y, Ma J, Cao D, Zhou Y, et al. (2018) Long non-coding RNAs in esophageal cancer: molecular mechanisms, functions, and potential applications. Journal of hematology & oncology 11(1): 118.
- Wang MW, Liu J, Liu Q, Xu QH, Li TF, et al. (2017) LncRNA SNHG7 promotes the proliferation and inhibits apoptosis of gastric cancer cells by repressing the P15 and P16 expression. European review for medical and pharmacological sciences 21(20): 4613-4622.
- Sun X, Huang T, Liu Z, Sun M, Luo S, et al. (2019) LncRNA SNHG7 contributes to tumorigenesis and progression in breast cancer by interacting with miR-34a through EMT initiation and the Notch-1 pathway. European journal of pharmacology PP. 856.
- Chi C, Li M, Hou W, Chen Y, Zhang Y, et al. (2020) Long Noncoding RNA SNHG7 Activates Wnt/beta-Catenin Signaling Pathway in Cervical Cancer Cells by Epigenetically Silencing DKK1. Cancer biotherapy & radiopharmaceuticals 35(5): 329-337.
- Han Y, Hu H, zhou J (2019) Knockdown of LncRNA SNHG7 inhibited epithelial-mesenchymal transition in prostate cancer though miR-324-3p/WNT2B axis in vitro. Pathology Research and Practice 215(10): 152537.
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8: 16.
- Zeng J, Ma YX, Liu ZH, Zeng YL (2019) LncRNA SNHG7 contributes to cell proliferation, invasion and prognosis of cervical cancer. European review for medical and pharmacological sciences 23(21): 9277-9285.
- Yang X, Sun L, Wang L, Yao B, Mo H, et al. (2019) LncRNA SNHG7 accelerates the proliferation, migration and invasion of hepatocellular carcinoma cells via regulating miR-122-5p and RPL4. Biomedicine and Pharmacotherapy p. 118: 109386.
- Cheng D, Fan J, Ma Y, Zhou Y, Qin K, et al. (2019) LncRNA SNHG7 promotes pancreatic cancer proliferation through ID4 by sponging miR-342-3p. Cell and Bioscience 9(1).
- Wu P, Tang Y, Fang X, Xie C, et al. (2019) Metformin suppresses hypopharyngeal cancer growth by epigenetically silencing long non-coding RNA SNHG7 in FaDu cells. Frontiers in pharmacology.
- Chi R, Chen X, Liu M, Zhang H, Li F, et al. (2019) Role of SNHG7-miR-653-5p-STAT2 feedback loop in regulating neuroblastoma progression. Journal of cellular physiology 234(8): 13403-134012.
- Luo X, Song Y, Tang L, Sun DH, Ji DG, et al. (2018) LncRNA SNHG7 promotes development of breast cancer by regulating MicroRNA-186. European review for medical and pharmacological sciences. 22(22):7788-7797.
- Chen Y, Peng Y, Xu Z, Ge B, Xiang X, et al. (2019) Knockdown of lncRNA SNHG7 inhibited cell proliferation and migration in bladder cancer through activating Wnt/beta-catenin pathway. Pathology, research and practice 215(2): 302-307.
- Li Y, Zeng C, Hu J, Pan Y, Shan Y, et al. (2018) Long non-coding RNA-SNHG7 acts as a target of miR-34a to increase GALNT7 level and regulate PI3K/Akt/mTOR pathway in colorectal cancer progression. Journal of Hematology and Oncology 11(1).
- Shan Y, Ma J, Pan Y, Hu J, Liu B, (2018) LncRNA SNHG7 sponges MIR-216b to promote proliferation and liver metastasis of colorectal cancer through upregulating GALNT1. Cell Death and Disease 9(7).
- Qi H, Wen B, Wu Q, Cheng W, Lou J, et al. (2018) Long noncoding RNA SNHG7 accelerates prostate cancer proliferation and cycle progression through cyclin D1 by sponging miR-503. Biomedicine and Pharmacotherapy 102: 326-327.
- Xia Q, Li J, Yang Z, Zhang D, Tian J, Gu B (2020) Long non-coding RNA small nucleolar RNA host gene 7 expression level in prostate cancer tissues predicts the prognosis of patients with prostate cancer. Medicine (United States) 99(7).
- Zhao D, Zhang H, Long J, Li M (2020) LncRNA SNHG7 functions as an oncogene in cervical cancer by sponging miR-485-5p to modulate JUND expression. OncoTargets and therapy 13:1677-1689.
- Wu F, Sui Y, Wang Y, Xu T, Fan L, et al. (2020) Long noncoding RNA SNHG7, a molecular sponge for microRNA-485, promotes the aggressive behavior of cervical cancer by regulating PAK4. Onco Targets and therapy 13: 685-699.
- Zhang Y, Yuan Y, Zhang Y, Cheng L, Zhou X, et al. (2020) SNHG7 accelerates cell migration and invasion through regulating miR-34a-Snail-EMT axis in gastric cancer. Cell cycle (Georgetown, Tex) 19(1): 142-152.
- Wang YH, Huo BL, Li C, Ma G, Cao W (2019) Knockdown of long noncoding RNA SNHG7 inhibits the proliferation and promotes apoptosis of thyroid cancer cells by downregulating BDNF. European review for medical and pharmacological sciences 23(11): 4815-4821.
- Lingling P, Cheng Y, Zou S, Song J (2019) Long noncoding RNA SNHG7 contributes to cell proliferation, migration, invasion and epithelial to mesenchymal transition in non-small cell lung cancer by regulating miR-449a/TGIF2 axis. Thoracic cancer 11(2): 264-276.
- Xu C, Zhou J, Wang Y, Wang A, Su L, et al. (2019) Inhibition of malignant human bladder cancer phenotypes through the down-regulation of the long non-coding RNA SNHG7. Journal of Cancer 10(2): 539-546.
- Chaudhry MA (2013) Expression pattern of small nucleolar RNA host genes and long non-coding RNA in X-rays-treated lymphoblastoid cells. Int J Mol Sci 14(5): 9099-9110.
- Zhou Y, Tian B, Tang J, Wu J, Wang H, et al. (2020) SNHG7: A novel vital oncogenic lncRNA in human cancers. Biomedicine and Pharmacotherapy 124.
- Zhang P, Shi L, Song L, Long Y, Yuan K, et al. (2020) LncRNA CRNDE and lncRNA SNHG7 are promising biomarkers for prognosis in synchronous colorectal liver metastasis following hepatectomy. Cancer management and research 12: 1681-1692.
- She K, Huang J, Zhou H, Huang T, Chen G, et al. (2016) LncRNA-SNHG7 promotes the proliferation, migration and invasion and inhibits apoptosis of lung cancer cells by enhancing the FAIM2 expression. Oncology reports 36(5): 2673-2680.
- Guo L, Lu J, Gao J, Li M, Wang H, et al. (2020) The function of SNHG7/miR-449a/ACSL1 axis in thyroid cancer. Journal of cellular biochemistry.
- Zhang L, Fu Y, Guo H (2019) C-Myc-induced long non-coding rna small nucleolar RNA host gene 7 regulates glycolysis in breast cancer. Journal of breast cancer 22(4): 533-547.
- Deng Y, Zhao F, Zhang Z, Sun F, Wang M (2018) Long Noncoding RNA SNHG7 Promotes the Tumor Growth and Epithelial-to-Mesenchymal Transition via Regulation of miR-34a Signals in Osteosarcoma. Cancer Biotherapy and Radiopharmaceuticals 33(9): 365-372.
- Wu X, Yuan Y, Ma R, Xu B, Zhang R (2020) LncRNA SNHG7 affects malignant tumor behaviors through downregulation of EZH2 in uveal melanoma cell lines. Oncology letters 19(2): 1505-1515.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...