Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5945
Case Report(ISSN: 2638-5945) 
Post-vaccination feline sarcoma, Report in Colombia Volume 4 - Issue 3
VM.Molina*1, J. Morales, MF1, Gutierrez2,3
- 1Boehringer Ingelheim, Pet Technical Service, Colombia
- 2Clinic veterinary Campomaskotas. Colombia
- 3University of La Salle, Colombia
Received:March 03, 2021 Published: April 26, 2021
Corresponding author: VM. Molina, Boehringer Ingelheim, Pet Technical Service, Colombia
DOI: 10.32474/OAJOM.2021.04.000190
Abstract
Post-vaccine feline sarcoma or injection site sarcoma (FISS) in a neoplasm described in felines in Colombia occurs after the use of inactive adjuvant vaccines. The objective is to describe the presence of a feline sarcoma after vaccination with inactive feline leukemia virus, first case described in Colombia. Un feline, male, half-breed, 7 years old, sterilized, vaccinated with inactive feline leukemia vaccine, for 3 years, develops a nodule 5 cm in diameter in the dorsal region, signature and non-allgesic. DeepE n dermis and striatum muscle evidence neoplastic proliferation of fusiform cells, mainly expansive and to a less invasive extent, organizing into short beams and long fascicles that intersect, with dense and lax spotlights and in spotlights is arranged in a fishbone pattern. The cells have fusiform morphology, slightly ovoid, irregular, some rounded, with faint and intense eosinophil cytoplasm in other denser spotlights. Cellular pleomorphism and moderate anistocytosis are evidenced. The nuclei have round, ovoid, and irregular morphology. It shows the presence of one or more nucléolos in some of its cells, mainly fine granular chromatin, nuclear pleomorphism and moderate anisocariosis. 4 mitosis were counted in 10 fields with the high power target at 2.37 mm2, no apparent lymphovascular invasion is observed, side edge compromise is evident. Additionally, discrete multifocal necrosis bulbs < 50%, multifocal moderate polymorphic polymorphonuclear inflammatory infiltrate and multifocal lymphocytic mononuclear infiltrate. Se describes the presence of post-vaccine feline sarcoma, as the first case described in Colombia.
Keywords: Feline; Neoplasm; Leukemia; Vaccine; Nodule
Introduction
In the United States and Europe,an increase in the presence of cats with post-vaccine sarcoma or feline injection site sarcoma (FISS) beginsto be discovered in 1992, and a link between the presence of sarcoma and the application of inactive feline leukemia and rabies vaccines [1,2,3]. Investigations made by [4] the incidence of post-vaccine feline sarcoma was estimated to be 1:1. 000 to 1:10. 000 cases; other descriptionsfound worldwide incidence of 3, 5:10. 000 cats vaccinated with inactive virus [5]; this is because vaccines with inactive viruses, require adjuvants to stimulate humoral response [6], finding significant statistical difference (P < 0,05), between adjuvant vaccines and those without adjuvants in the presence of sarcoma. The main adjuvant used is Aluminium present in inactive vaccines, intheform of Aluminium Hydroxide or Aluminium Phosphate. Thesestimulatethe proliferationof proinflammatory cells, especially macrophages, which predisposes to chronic inflammatory changes, responsible for cell mutagenesis [1]. It should be noted that there are other factors that favor the de velopmentof post-vaccine feline sarcoma, such as the simultaneous application of several vaccines at the same site or the use of vehicles in the formulation of parenteral drugs, which are also responsible for the development of neoplasm, such as hydrochloride, trihydrate and acetate, which are often used in antibiotics and long-acting anti-inflammatory drugs [6,7,8,9,10]. Genetic changes caused by cellular alterations, due to crónicinflammation, favor the activation of oncogenes inducing mesenchymatous neoplasm; therefore, any chronic inflammatory process in a feline can induce sarcoma [6,11]. FiSS, with malignancy characteristics, is known to be highly infiltrative locally, high growth rate, to gresive and metastatic potential; therefore, they require extensive excision, which may include member amputation [7-12,13] it is known that the MOSTcommonly described FISS are fibrosarcoma, chondrosarcoma, liposarcoma, rhabdomyosarcoma and osteosarcoma [14,15]; which can be solid or cystic, mobile or fixed and can sometimes exceed 3-4 cm in diameter [16] and ebony to post-vaccine feline sarcomas, have low incidence, possibly due to individual genetic characteristics, particular immune reactions and the presence of adjuvants in inactive vaccines [6] the description is relevant, because the sarcoma asassociated with the injection siteare different in pathology and biological behavior against sarcomas not associated with the injection site [17]. Thedifference between sarcomas not associated with the injection site and postvaccine sarcoma (FISS),is that the former have a larger size and local recurrence rate of 30%,with respect to the smallest seconds and with greater recurrence 70% [13-18,19] . In addition, she has indicated that feline leukemia virus (ViLeF) and immunodeficiency (VIF), son some of those responsiblefor non-vaccine sarcomas, such as lymphosarcoma [20] and fibrosarcoma (FS), caused by feline sarcoma virus (FeSV), which appears after recombination of the FeSV genome with cellular oncogenes, which are very aggressive locally and with high metastatic capacity, while postvaccine sarcomas, are solitary neoplasms and fisS has not been related to ViLeF and FeSV [21] but the presence of both should always be ruled out when asarcoma appears, for this reason currently the guidelines for the application of inactive vaccines in felines (WASA; World Small Animal Veterinary Association; AAFP; American Association of Feline Practitioners; ABCD; Advisory Board on Cat Diseases VAFSTF; Grupo working on feline sarcoma associated with the vaccine) indicates that it should be applied at the base of the tail or hind limbs [5-11] and not interscapular, because post-vaccine sarcoma requires extensive surgical excision [6-23]. Feline injection site sarcoma (FISS) is described in a halfbreed cat following the use of inactive feline leukemia vaccine and linking its low description in Colombia to the genetic interactions responsible for the development of FISS in other countries [3-15], in addition, to describe for the first time a related case in Colombia.
Materials and Methods
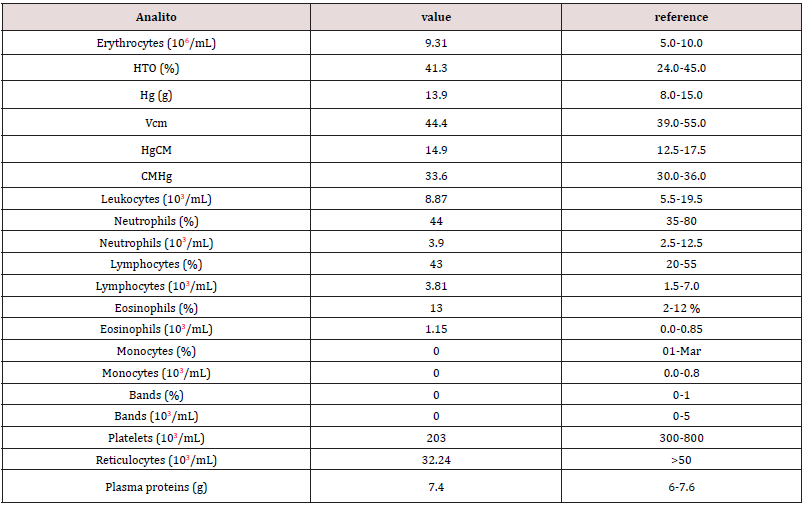
Figure 1: Feline patient with a nodule 5 cm in diameter, in the dorsal region after interscapular space (red circle).

Anamnesis: a half-breed feline, 7 yearsold, sterilized, came to consultation by presenting a nodule 5 cm in diameter, in the dorsal region, flow into the interscapular space (Image 1), after vaccination against feline leukemia, with virus inactive for 72 months, the patient received annual vaccination against feline infectious rhinotracheitis, calicivirosis and panleucopenia (attenuated virus) and rabies (inactive) in the right posterior member as indicated by the WASAVA and AAFP vaccination guidelines [24-26] plus multiple doses of feline leukemia (inactive virus) in the dorsal (interscapular) region; being the last vaccine 12 months ago, it is described that the application of inactive ViLeF, was always performed in the same region.
Clinical examination: sterilized male feline patient, who has a painless nodule in the dorsal region, which is ulcerated (Image 2), an active and dynamic patient was observed at the clinical examination, attention to the medium, body condition 3/5, heart rate 100 palpitations per minute, breathing rate 40 breaths per minute, temperature 39,4oC, capillary filling time, 2 segundos,wet oralmucosa, pink, pulse 102 pulsaciones por minuto,strong, high and rhythmic arc, skin fold 1 segundos.
Figure 2: Ulcerative lesion in the dorsal region, 5 cm in diameter with circumscribed alopecia (Red Circle).
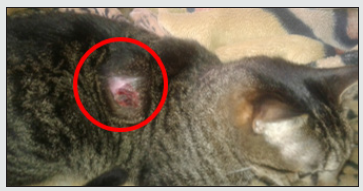
Hemoleucogram: The feline patient underwent a blood sample, external jugular vein after antisepsis procedure, to evaluate full hematic profile, the hemoleucogram was processed into HA 22 Touch Vet® (Clindiang Systems©, China, Zhenjiang) is evaluated erythrocytes, leukocytes, neutrophils, eosinophils, lymphocytes, bands, monocytes, thrombocytes, hematocrit (HTO), hemoglobin (Hg), mean corpuscular volume (CMV), mean corpuscular hemoglobin (HgCM), mean haemoglobin concentration (CMHg) and plasma proteins (Table 1).
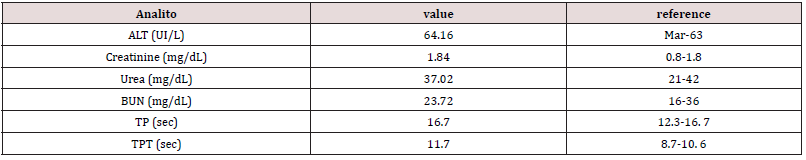
Blood chemistry: The patient underwent basic profile tests, alanine amino transferase (ALT), alkaline phosphatase (ALP), creatinine and urea, in a Mindray BS-240® (Mindray Medical International Limited, Shenzhen, China) chemical analyzer equipment, the sample was taken from the subsequent asepsis external jugular vein, the values found can be seen in Table 2.
Table 2: Basic liver-renal profile with clotting times in patients with fibrosarcoma, the first day of medical consultation, before surgery.
Viral Test: the patient underwent immunodiagnosis testing (IDEXX Laboratories©, Snap Combo® VIF/ViLeF, Toronto, Canada), which was negative for both viruses; but to determine the presence of ViLeF in regressive phase was also performed PCR for ViLeF, VIF, Mycoplasma haemofelis and Bartonella spp, resulting in all negative tests in reference laboratory, Medellin, Colombia.
Ultrasound: The patient underwent full abdominal ultrasound in search of metastasis of neoplastic injury, the abdominal cavity was evaluated, without the presence of effusions or reactive lyphonates. In gastrointestinal evaluation, stomach with wall of preserved thickness, low free hyperecoic content, moderate hypoecocal content, no pressure pain, small intestine with preserved thickness wall, correct motility, low gas content, moderate fecal content, colon with preserved thickness wall, moderate fecal content, moderate gas content, no obstructivesigns, spleen, hypoecocal parenchyma, fine dotted, homogeneous, sharp edges, preserved size,if n presence of focal lesions and preserved vasculature; liver with hypoecoic parenchyma, thick, homogeneous granules, rounded edges, hyperecoetic capsule, preserved size, no focal, cystic or nodulal lesions were evident; gallbladder, moderate homogeneous anechoic content,preserved walls, no obstructivesigns; kidneys exhibited regular contour, preserved size and shape, 1:1 corticomedular ratio, correct corticomedular differentiation, homogeneous ecogenicity, no obstructive signs, no pain; the pelvis dilated at the left and bladder level, moderate homogeneous anechoic content, preserved walls, no obstructivesigns; no presence in the abdominal cavity of masses or elements indicating the presence of metastasis (Image 3). X-ray: a chest latero-lateral radiographic plate is performed, in which an injury of 5 cm long at the dorsal level is observed, between T10-T12 (Image 4); in the image it can be observed that the lesion is not infiltrating the muscle tissue, only located in the superficial dermal tissue.
Figure 3: Complete abdominal ultrasound, kidney, bladder, liver, biliary vesicular, can be observed without pathological changes
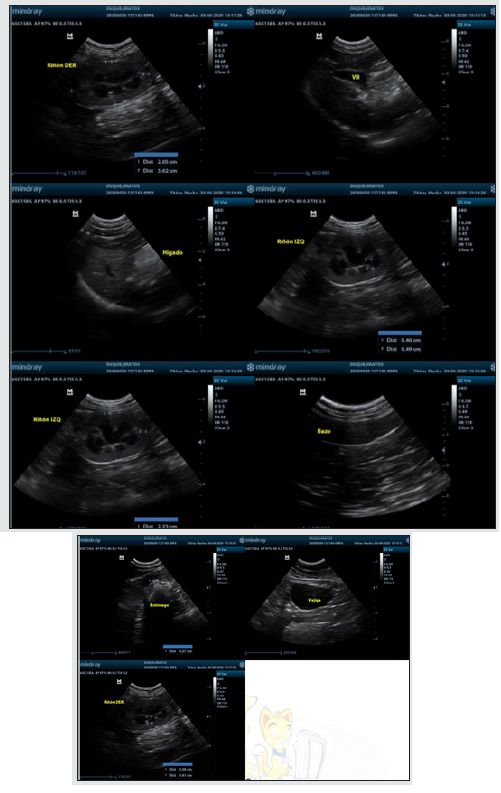
Figure 4: Latero-lateral chest radiographic plate, in which you can see a radiopaca structure between T10-T12 (white arrow) in the dorsal region not attached to the bone tissue.
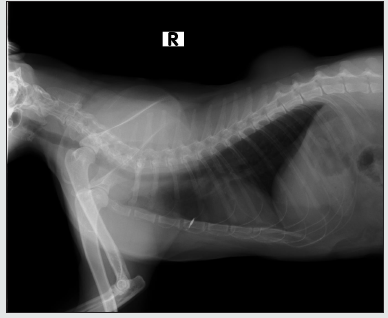
Figure 5: Cytology of the dorsal nodule, with PAAF technique, staining with Wright, observation 10X, where you can observe.
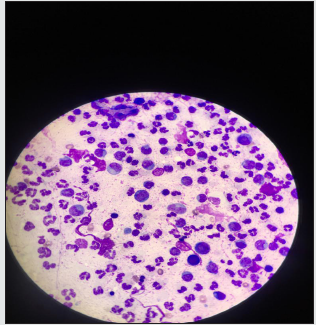
Cytology: Fine needle aspiration (PAAF) puncture was performed,for cell evaluation of the nodule, thenWright staining, were observed under the microscope at 10 X, lymphocyte proliferation and some macrophage polymorphs (Image 5). It is observed that there is severe mixed infiltrate of PMN predominance, presence of fusiform morphology cells, agglomerates of circular morphology cells, some fusiform to oval, anisocytosis and mild anisocariosis and moderate hyperchromasia.
Results
The patient undergoes surgical procedure to excision of the neoformation, with the following surgical procedure.
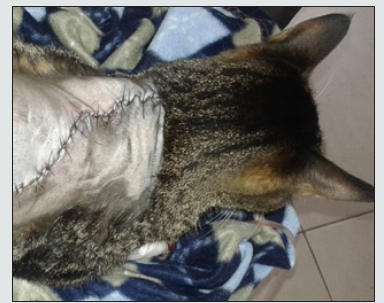
Surgery: The patient undergoes a surgical excision of the lesion (Image 6 ), under anesthesia with isoflurane 1,5 CAM, is induced with propofol 6 mg iv, premedical with xylacin 1,5 mg sc, acepromacin 0,15 mg sc, tramadol 6 mg iv,meloxicam 0,6 mg iv and cephalotin 60 mg iv and was treated post-surgically with cephaloxin 60 mg every 12 hours, po, meloxicam 0,3 mg once daily, po; disinfection chlorhexidine,leaving the patient hospitalized for 5 days, was taken 4 pieces of tissue, one of the fragments is referenced with suture. They have irregular shape, dimensions of 0,6*0,4*0,4 cm to 1,5*0,5*1,0 cm. The fragment that presents the suture shows skin and hair on one of its surfaces, the fragments have beige coloration and semi-soft consistency. Tissue with surgical suture is referenced with ink, preserved in 10% formaldehyde, for histopathological evaluation, sent to reference laboratory; Medellin, Colombia; cuts are made to each of the fragments and forwarded to processing.
Figure 6: Feline after the intervention of excision of the dorsal nodule, in which moreic removal of the circulating tissue had to be performed.
Histopathology: In the microscopic evaluation of fragments, neoplastic proliferation of fusiform cells (Image 6) was evident, mainly expansive and to a less invasive extent, organizing into short beams and long fascicles that intersect, with dense and lax spotlights and in spotlights is arranged in a fishbone pattern (Image 7). The cells had fusiform morphology, slightly ovoid, irregular, some rounded, with faint and intense eosinophil cytoplasm in other denser spotlights. Cellular pleomorphism and moderate anistocytosis are evidenced. The nuclei had round, ovoid, and irregular morphology. The presence of 1 or more nucléolos in some of its cells, mainly fine granular chromatin, nuclear pleomorphism and moderate anisocariosis, was evident. 4 mitosis were counted in 10 fields with the high power target at 2.37 mm2, no apparent lymphovascular invasion was observed, side edge compromise was evident (Image 7). Additionally, discrete multifocal necrosis spotlights less than 50%, polymorphophilic polymorphonuclear mixed inflammatory infiltrate, moderate multifocal and multifocal lymphocytic mononuclear infiltrate.
Figure 7: In the observed histological cut 4 X; a proliferation of basophilic (Arrow) cells can be observed.
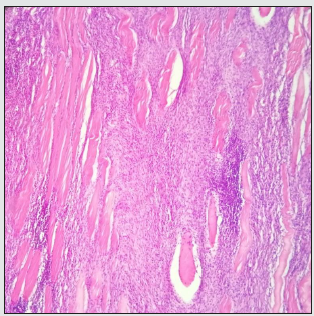
Severe deposition of collagen fibers from elongated to oval morphology is observed, some forming thick acellular agglomerates and others intermingled and upholstered among abundant cellularity. An organization pattern is evident in undulating fascicles.
Discussion
The presence of fibrosarcomas in felines caused by the application of drugs or vaccines (FISS),is not very welldescribed in Colombia, although there are several research papers on the presence of these tumors in cats there is no report of FISS in a Colombian feline, this may be due to the low frequency 1-3,6:10000 [27,28]; which does not indicate that it does not exist, but has not been documented; it is known that there are oncogenes related to frequency, but possibly because they are half-breed cats in Colombia, the presence of these are low in Colombian felidos, compared to purebred felines. It is known that vaccines’ relationship with FISS development has been well described since 1992, which describes some association between ViLeF vaccination and rabies with the development of sarcomas in cats, as in the case, where the cat receives multiple doses of inactive ViLeF at the same site for years, with an increased risk of up to 5 times for rabies and 2 times for ViLeF [8-29] although it has been described that antirubbic, as the largest responsible [3] in ourcase is ruled out because it was placed in the later member; ViLeF vaccine is known to be inactive,has aluminium hydroxide as an adjuvant, responsible for chronic inflammation [3-50] vaccine that was describedin thiscase, in addition, multipleinvestigations showed relationship between number of doses andn the same siteand the presence of neoplasm, being only 50% for a single dose, 127% for 2 doses of vaccines and 175% for three or more vaccines [2-16, 47] as described in the case of three consecutive doses per year. Although other vaccines have also been associated as causes of FISS [4-19,30] in this case this possibility is ruled out because these were administered in the later right member. Other research indicates that vaccines adjuvantedwith aluminio do not always produce postnalreaction, but if they are most frequently responsible [8-15,31]. In addition, other substances may carry FISS, but in this case feline was not treated with drugs containing hydrochloride, acetate or trihydrate in the last 3 years [7-29,32] and although the feline had microchip another cause of FISS [43-46], this is located in the esternal region and the possibility that FISS will be developed by microchip, is onlyr that by repetitive vaccination with inactive ViLeF, administered for 3 years and nthe samesite, coinciding with what is described by other authors [2-16]. L to FISS relationship with ViLeF presence is described as not existing [8-13] but if there is sarcoma by ViLeF,so the presence of the disease is ruled out; viLeF and FeSVinduced neoplasms account for 33,3% of neoplasms in cats and 62% of T-cell lymphoma [20], in addition to other sarcomas such as: neuroblastomas, osteochondromas and fibrosarcomas [49] activation of MYC and P53 oncogenes [18-26] and although FISS is not closely related ViLeF, feSV is known to cause feline sarcoma [44];therefore the feline was negative to test p27 and to PCR for ViLeF, which derails neoplasm of viral origin; but it should be noted that the activation of the p53 oncogen, can occur without the presence of ViLeF or FeSV, more often in pure cats than in mixed felines, that explains the low description of the problem in Colombia, where there is a high population of mixed cats. The type neoplasia of this case is a fibrosarcoma, which is often, 50% of cases [1], continues with malignant fibrous histiocytoma 28,2% [1] (Hendrick and Brooks, 1994), osteosarcoma, chondrosarcoma, liposarcoma, rhabdomyosarcoma, and undifferentiated sarcoma [5- 32] The description of fibrosarcoma showing poorly differentiated neoplastic cells in the form of a spindle, elongated pleomorphic nuclei, lymphocyte infiltrate and mitosis; which coincides with the description of feline patient neoplasm (Image 7-8); malignant, originating from fibroblast, in the subcutaneous tissue of cats with an average age of 12 years [40],which in the case is lessthan 7 years old, fibrosarcoma accounts for 12-25% of feline skin tumors [17] is located on the trunk and subsequentxtremities [40], in the case presented on the back of the application site. They are neoplasms of local recurrence, but little metastatic [39-40] whichis confirmed by the radiology and ultrasound report, see images 3-4 respectively. While fixed needle aspiration (PAAF) puncture analysis, inflammatory infiltrate composed of lymphocytes and macrophages was presented along with an increase in mitotic index [32] as you can see in the case (Image 4), where the lymphocytes are the predominant69s,2%, as can be seen in Figure 4, which coincides with the description of several authors [17] although PAAF has been considered not ideal, as it generates confused diagnosis with a granuloma [11], therefore the definitive diagnosis is only by bincisional iopsia [6-22]as was done in the patient.
Laboratorytests in general are consideredndo not have much diagnostic value in FISS [13] it is known that the tests will determine the overall health of the patient; even if there is no association between FISS and hematology [13-21]. In the present case it could be observed that both the hemoleucogram (Table 1), as well as the liver and renal profile (Table 2), were found within normal values, which coincides with others, where FISS does not affect haematological or biochemical analysis [13]. Theidentification of FISS is mostly given by the presence of a postvaccine nodule,whichis confused with abscesus or seroma [23] therefore, the history of vaccination, site and type of vaccine [5], this information was important in the present case,several doses at the same site, predisponent factor [11], in addition to inactive viruses with aluminum as an adjuvant as in the case. As for the treatment of FISS, you should consider surgery to be the basic pillar of FISS treatment, a wide surgical removal of the primary tumor, radical excision [11-32]; because patients not undergoing radical excision have recurrence of the injury within 10 months [14-42]; condition that occurs in the patient, in which an extensive excision was performed (Image 6); following the indication of other authors, who suggest that tumors > 5 cm in diameter, require extensive splits [16-33, 34]. Chemotherapia that destroys high division cells [35] should not be considered for definitive treatment against FISS, but can be palliative, avoiding metastasis [36] the use of chemotherapy surgery has yielded good results in tumor control and prolongation of life expectancy [5-16]. Several chemotherapeutic agents have been shown to have activity against FISS, including doxorubicin, carboplatin, cyclophosphamide, ifosfamide and vincristine [22- 37] for the present case the patient was only subjected to surgical and non-pharmacological treatment and as for the established treatment protocol it is known that extensive surgery relatively improves prognosis [38] which can be seen in the patient described, where the excision of neoplasm was wide, to avoid relapses (Image 6). This is because histological malignancy considerations such as tumor necrosis, cell pleomorphism, mitotic activity, cellularity, degree of tumor differentiation and amount of stromal, describes them as low grade or intermediate grade [13-54].
Conclusion
Inactive vaccines using adjuvants pose a latent risk in the presence of post-injection feline sarcomas, the presence of feline sarcoma caused by the application of an inactive feline leukemia vaccine is described for the first time in Colombia.
References
- Hendrick MJ, Brooks JJ (1994) Postvaccinal sarcomas in the cat: histology and immunohistochemistry. Veterinary Pathology 31(1): 126-129.
- Kass PH, Barnes WG, Spangler WL, Chomel BB, Culbertson MR (1993) Epidemiologic evidence for a causal relation between vaccination and fibrosarcoma tumorigenesis in cats. JAVMA 203(3): 396-405.
- Woodward K (2011) Origins of injection- site sarcomas in cats: the possible role of chronic inflammation- A Review. ISRN Veterinary Science 10 p. : 1-16.
- Hendrick MJ, Goldschmidt MH, Shofer FS, Wang YY, Somlyo AP (1992) Postvaccinal sarcomas in the cat: Epidemiology and electron probe microanalytical identification of aluminum. Cancer Research 52(19): 5391-5394.
- Skorupski KA, Shaw SC, Kent MS, Gordon IK, Collins CJ, et al. (2009) Temporal changes in characteristics of injection-site sarcomas in cats: 392 cases (1990-2006). JAVMA 234(3): 376-380.
- Séguin B (2002) Injection site sarcomas in cats. Clinical Techniques in Small Animal Practice 32(4): 983-985.
- Buracco P, Martano M, Morello E and Ratto A (2002) Vaccine- associated- like fibrosarcoma at the site of a deep non-absorbable suture in a cat. The veterinaryjournal 163(1): 105-107.
- Kirpensteijn J (2006) Feline injetion site- associated sarcoma: Is it a reason to critically evaluate our vaccination polices? Journal of veterinary Microbiology 117(1): 59-65.
- Macy DW (1999) Current understanding of vaccination site-associated sarcomas in the cat. Journal of Feline Medicine and Surgery 1(1): 15-21.
- McLeland SM, Imhoff DJ, Thomas M, Powers BE and Quimby JM (2013) Subcutaneous fluid port-associated soft tissue sarcoma in a cat. Journal of Feline Medicine and Surgery 15(10): 917-920.
- Martano M, Morello E and Buracco P (2011) Feline injection- site sarcoma: Past, present and future perspectives. Veterinary Journal 188(2): 136-141.
- Hartmann K, Day MJ, Thiry E, Lloret A, Frymus T et al. (2015) European Advisory Board on Cat Diseases. Feline injection site sarcoma: ABCD guidelines on prevention a management. Journal of Feline Medicine and Surgery 17(7): 606- 613.
- Morris J and Dobson J (2001) Small Animal Oncology, USA, Editorial Offices. p. 304.
- Hershey AE, Sorenmo KU, Hendrick MJ, Shofer FS, Vail DM (2000) Prognosis for presumed feline vaccine associated sarcoma after excision: 61 cases (1986–1996). JAVMA 216(1): 58-61.
- Macy DW, Hendrick MJ (1996) The potential role of inflammation in the development of postvaccinal sarcomas in cats. Vet Clin Small Anim 26(1): 103-109.
- Dobson JM, Demetriou JL (2014) Skin tumors. China. Saunders Elsevier. 768 p.
- Doddy FD, GlickmanLT, Glickman NW, Javovitz EB (1996) Feline fibrosarcomas at Vaccination Sites and Non- vaccination Sites. Journal of Comparative Pathology 114(2): 165-174.
- Almimira J, Villafranca M (1998) Skin oncology. Canis et felis 32: 10-46.
- Bregazzi VS, Larue SM, McNeil E, Macy DW, Dernell WS, et al. (2001) Treatment with a combination of doxorubicin, surgery, and radiation versus surgery and radiation alone for cats with vaccine-associated sarcomas: 25 cases (1995–2000). JAVMA 218(4): 547-550.
- Molina VM (2013) Mediastyn lymphoma by feline viral leukemia. J. Agr. Anim. Sci 2(1): 80-86.
- Nagell M (1998) Fibrosarkome der Katze. Prakt Tierarzt 79: 600-606.
- Magden E, Quckenbush SL, Vandewoude S (2011) IVF associated neoplasms-A mini Review. Veterinary Immunology and Immunopathology 143(3): 227-234.
- Bowlt K (2015) Feline injection site- associated sarcomas. In practice 37(1): 2-11.
- Couto SS, Griffey SM, Duarte PC, Madewell BR (2002) Feline vaccine-associated fibrosarcoma: morphologic distinctions. Veterinary Pathology 39(1): 33-41.
- Day MJ, Horzinek MC and Schultz RD (2010) WSAVA guide- lines for the vaccination of dogs and cats. Journal of the Small Animal Practice 51 p. : 338-356.
- Richards JR, Elston TH, Ford RB, Gaskell RM, Hartmann K, et al. (2006) The 2006 American Association of Feline Practitioners feline vaccine advisory panel report. JAVMA 229(9): 1405-1441.
- Scherk MA, Ford RB, Gaskell RM, Hartmann K, Hurley KF, et al. (2013) AAFP Feline Vaccination Advisory Panel Report. Journal of Feline Medicine and Surgery 15(9): 785-808.
- Day MJ, Schoon HA, Magnol JP, Saik J, Devauchelle P, et al. (2007) A kinetic study of histopathological changes in the subcutis of cats injected with non-adjuvanted and adjuvanted multi-component vaccines. Vaccine. 25(20): 4073-4084.
- Kliczkowska K, Jankowska U, Jagielski D, Czopowicz M, Sapierzynski R (2015) Epidemiological and morphological analysis of feline injection site sarcomas. Journal of Veterinary Sciences 18(2): 313- 322.
- Kass PH, Spangler WL, Hendrick MJ, McGill LD, Esplin DG, et al. (2003) Multicenter case-control study of risk factors associated with development of vaccine-associated sarcomas in cats. JAVMA 223(9): 1283-1292.
- Lester S, Clemett T, Burt A (1996) Vaccine site-associated sarcomas in cats: clinical experience and a laboratory review (1982–1993). JAAHA 32(2): 91-95.
- Gobar GM, Kass PH (2002) World Wide Web-based survey of vaccination practices postvaccinal reactions, and vaccine-site associated sarcomas in cats. JAVMA 220(10): 1477-1482.
- Hauck M (2003) Feline injection site sarcomas. Vet Clin Small Anim 33(3): 553-571.
- Ladlow J (2013) Injection site associated sarcoma in the cat treatment recommendations and results to date. Journal of Feline Medicine and Surgery 15(2): 409-418.
- Phelps HA, Kuntz CA, Milner RJ, Powers BE, Bacon NJ (2011) Radical excision with five-centimeter margins for treatment of feline injection-site sarcomas: 91 cases (1998-2002). JAVMA 239(1): 97-106.
- Nelson RW and Couto GC (2020) Small Animal Internal Medicine. 6th Ed. St Louis. Elsevier. 1504 p.
- Giudice C, Stefanello D, Room M, Cantatore M, Russo F, et al. (2010) Feline injection-site sarcoma: recurrence, tumour grading and surgical margin status evaluated using the three dimensional histological technique. The VeterinaryJournal. 186(1): 84-88.
- Hill J, Lawrence J, Saba C, Turek M, Feldhaeusser B, et al. (2014) In vitro efficacy of doxorubicin and etoposide against a feline injection site sarcoma cell line. Research in Veterinary Science 97(2): 349-357.
- Blessed M (1997) Chondrosarcoma in the cube and radius of a cat. Clinical case. AVEPA 17(4): 207-209.
- Folgearini MS, Bonel JS, Machado P, Gevehr CF (2015) Soft tissue sarcomas in canines and felines: ep ideologicaland pathological aspects. Rev Veterinary Sciences 31(2): 7-12.
- Liptak JM, Forrest LJ (2013) Soft tissue sarcomas. In: Withrow S, Vail D and Page R. Small Animal Clinical Oncology. 5th Ed. St Louis. Saunders. p. 425-454.
- Carwardine D, Friend E, Toscano E and Bowlt K (2014) Owner preferences for treatment of feline injection site sarcomas. Journal of Small Animal Practice 55(2): 84-88.
- Davidson EB, Gregory CR, Kass PH (1997) Surgical excision of soft tissue fibrosarcomas in cats. Veterinary Surgery 26(4): 265-269.
- Daly MK, Saba CF, Crochik SS, Howerth EW, Kosarek CE, et al. (2008) Fibrosarcoma adjacent to the site of microchip implantation in a cat. Journal of Feline Medicine and Surgery 10(2): 202-205.
- Ellis JA, Jackson ML, Bartsch RC, McGill LG, Martin KM, et al. (1996) Use of immunohistochemistry and polymerase chain reaction for detection of oncornaviruses in formalin-fixed, paraffin-embedded fibrosarcomas from cats. JAVMA 209(4): 767-771.
- Martano M, Morello E, Lussich S and Buracco P (2012) A case of feline injection- site sarcoma at the site of cisplatin injections. Journal of Feline Medicine and Surgery 14(10): 751-754.
- Miller MA, Aper RL, Fauber A, Blevins WE, Ramos-Vara JA (2006) Extraskeletal osteosarcoma associated with retained surgical sponge in a dog. Journal of Veterinary Diagnostic Investigation 18(2): 224-228.
- Morrison WB and Starr RM (2001) Vaccine- Associated Feline Sarcoma Task Force: Vaccine-associated feline sarcomas. JAVMA 218(5): 697-702.
- Nimwegen V and Kirpensteijn J (2012) Specific disorders In Veterinary Surgery Small Animal. Ed.M. Johnston, S. A. Tobias. Saunders 765 p.
- Pool RP, Carrig CB (1972) Multiple cartilagenous exostoses in a cat. Veterinary Pathology. 9: 350-359.
- Sinkovics JG (2007) Adult human sarcomas. I. Basic science. Expert Review of Anticancer Therapy 7(1): 31-56.
- Carminato A, Vascellari M, Marchioro W, Melchiotti E and Mutinelli F (2011) Microchip-associated fibrosarcoma in a cat. Veterinary dermatology 22(6): 565-569.
- London CA, Hannah AL, Zadovoskaya R, Chien MB, Kollias Baker C, et al. (2003) Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clinical Cancer Research 9(7): 2755-2768.
- Romanelli G, Marconato L, Olivero D, Massari F, Zini, E (2008) Analysis of prognostic factors associated with injection-site sarcomas in cats: 57 cases (2001-2007). JAVMA 232(8): 1193-1199.
- Vascellari M, Melchiotti E, Bozza MA, Mutinelli F (2003) Fibrosarcomas at presumed sites of injection in dogs: characteristics and comparison with non-vaccination site fibrosarcomas and feline postvaccinal fibrosarcomas. JAVMA 50(6): 286-291.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...