Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5945
Case Report(ISSN: 2638-5945) 
Deletion of 9q Causes Chronic BCR-ABL Negative Benign Neutrophilia Volume 2 - Issue 5
Cherian Verghese*1, Xiaojin Sha2, Talal Khan1, Emmanouil Alimpertis1, Hany Meawad2, Riddhish Sheth2, Feraz Khogeer1, Anis Toumeh1, Nauman Siddiqui3 and Robert Booth2
- 1 Division of Hematology & Oncology, University of Toledo, USA
- 2 Division of Pathology, University of Toledo, USA
- 3 Tufts University School of Medicine, USA
Received: May 03, 2019 Published: May 13, 2019
Corresponding author: Cherian Verghese, Division of Hematology & Oncology, University of Toledo, USA
DOI: 10.32474/OAJOM.2019.02.000149
Abstract
Background: Partial deletions of the long arm of chromosome 9 are associated with various congenital structural and functional defects, developmental delay, mental retardation as well as neuropsychiatric abnormalities. Interstitial deletion of 9q is also a recurring abnormality in malignant myeloid diseases including AML and rarely associated with ALL as well. We present a case of 9q deletion associated with benign leukocytosis in an adult patient.
Case presentation: The case involves a 49-year-old female who presented to our center with a 6-year history of unexplained leukocytosis. Bone marrow evaluation showed metaphase cells to have a deletion of 9q. Bone marrow was also negative for any features of dysplasia or increase in blast cells. The peripheral blood was negative for both JAK2 mutation and BCR-ABL fusion gene product.
Conclusion: Isolated 9q deletion can present as benign chronic neutrophilia. This should be considered after ruling out more common causes of neutrophilia including chronic infections, inflammation, certain medications, hemorrhage, splenectomy, hemolytic anemia and malignant myeloid disorders.
Keywords: 9q Deletion; Benign neutrophilia
Introduction
Leukocytosis is defined as an elevation in the WBC count that is inappropriate for the patient’s age. WBC counts of 30 X 109 /L may be seen normally in infants but is abnormal in an adult. The normal WBC differential also changes one ages with greater absolute numbers of both neutrophils and lymphocytes seen usually only in newborns. The lymphocyte count continues to stay high throughout childhood [1]. Myeloid leukocytosis may in turn involve the granulocytes (ie. Neutrophilia, Eosinophilia or Basophilia) or agranulocytes (Lymphocytosis or Monocytosis). Neutrophilia is defined as an agereferenced elevated circulating neutrophil count greater than 7.7 X 109 /L in adults. In general, neutrophilia may be reactive or clonal. Reactive neutrophilia or leukemoid neutrophil leukocytosis may result from an increased production, de-margination or decreased egress into tissues from the intravascular space. Leukemoid neutrophil leukocytosis is commonly seen in infections. However, any significant stimulation of the bone marrow may elevate the neutrophil count. This may include malignancies, medications, colony stimulating factors, inflammations, hemorrhage and splenectomy. It may also be seen in conditions as diverse as diabetic ketoacidosis and alcoholic steatohepatitis [2,3]. Malignant or clonal elevations in the neutrophil count can be seen in chronic myelogenous leukemia, myelodysplastic/ myeloproliferative disorders, chronic neutrophilic leukemia and acute myeloid leukemia. In primary malignant processes the counts are more likely to be greater than 50 X 109 / L. With a leucoerythroblastic picture there is predominance of blasts and this may result from both primary marrow pathologies well as infiltrative marrow processes. It is important to note that use of colony stimulating factors may also be associated with a leukoerythroblastic picture. Morphological that indicate a benign leukemoid processes include Döhle bodies, toxic granulations, cytoplasmic fragments, thrombocytosis and eosinophilia. Unlike eosinophilia which is usually reactive, the presence of basophilia should prompt a bone marrow study to rule out a primary myeloid malignant process. It is also uncommon for a benign neutrophilic leukocytosis to be associated with any molecular or cytogenetic abnormalities. The most common molecular marker of a malignant leukocytosis is the BCR-ABL fusion gene on molecular testing or the 9:22 translocation on cytogenetic evaluation. Partial or complete deletions of 9q have not been described as presenting with benign leucocytosis. We describe here a case of BCR-ABL negative leukocytosis with an isolated 9q deletion.
Case Presentation
A 49-year-old female presented to our facility with a 6-year history of stable unexplained leukocytosis involving the neutrophil cell line (Table 1). Medical history was relevant only for systemic hypertension. Clinical complaints included moderate fatigue and occasional constipation. There was no weight loss, bleeding or bruising. She denied taking any steroids and was only using acetaminophen and ibuprofen. She had been smoking less than ½ a pack a day for nearly 20 years and had been worked up previously for a possible infectious process. Despite the work-up being negative, she had been given multiple courses of antibiotics. Physical examination did not reveal any congenital structural defects, splenomegaly, lymphadenopathy, rash, signs of infection or inflammation. CT imaging of the abdomen and pelvis was negative for any lymphadenopathy or splenomegaly.
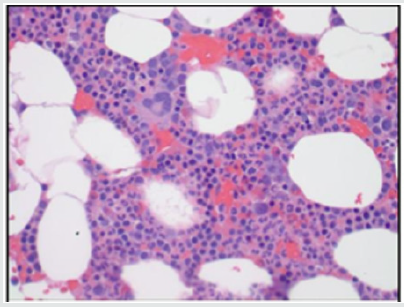
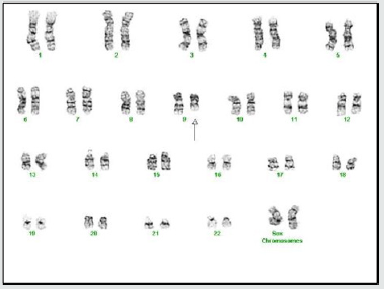
A complete blood count showed a WBC count of 22 X 109 /L with 73% neutrophils. The rest of the CBC was normal and included an RBC count of 4.94mil/mm3 , a Hemoglobin of 14.1g/dl and a platelet count of 252,000/mcL. Differential count showed lymphocytes at 23.1%, monocytes at 6.2%, eosinophils at 4.7% and basophils at 0.4%. The peripheral blood smear showed morphologically normal WBC’s. Serum Immunoglobulin levels were normal. Bone marrow aspirate revealed normal cellularity with trilineage hematopoiesis without any evidence of dysplastic or neoplastic features (Figure 1(a-d)). The marrow cell count showed neutrophils at 30%, eosinophils at 2% and monocytes at 1%. Plasma cells were not increased (1%), lymphocytes were 12% and nucleated erythroid cells at 20%. Flow cytometry revealed blasts at only 1%, lymphocytes at 10% and monocytes at 3%. Myeloid cells were the dominant cell line at 82% (Figure 2). Cytogenetic analysis showed that two of twenty metaphase cells had a deletion of the long arm of chromosome 9 (q13-q22). No other clonal numerical or structural abnormality was observed (Figure 3). The FISH analysis for BCRABL fusion gene product was negative in the peripheral blood. We examined two hundred interphase nuclei and the signal pattern obtained did not differ from normal controls. Repeat testing for the fusion of BCR-ABL with RT-PCR came back negative as well. The PCR analysis was negative for a JAK2 V617F point mutation in the peripheral blood.
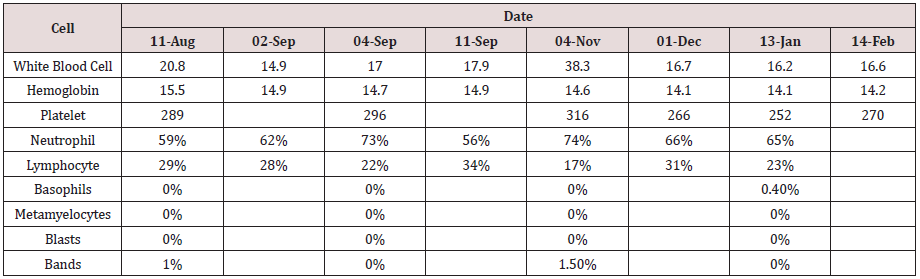
Figure 1(a): Low power magnification of bone marrow aspirate reveals a normocellular marrow. (Hematoxylineosin stain, original magnification  40).
40).
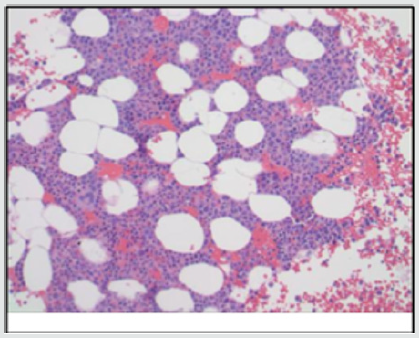
Figure 1(c): CD34 immunostain showing only few scattered blasts which were confirmed by flow as <5%.
Figure 2: Flowcytometry of bone marrow aspirate showing <1% CD34 blast population, a normal myeloid maturation pattern and a polyclonal B-cell population. No evidence of any aberrant immunophenotype based on Kappa, Lambda, HLA-DR, CD34, CD117, CD11b, CD16, CD13, CD14, CD33, CD7, CD64, CD56, CD3, CD57, CD8, CD4, CD2, CD103, CD10, CD20, CD38, FMC-7, CD23, CD22, CD5, CD19 antibody flowcytometric profile.
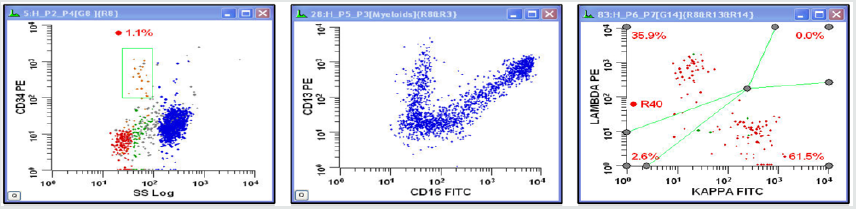
Figure 3: Level 350 G-banding of bone marrow cells
showing a 46, 
 , del(9) (q13q) Karyotype in 3 of 17
metaphase cells analyzed.
, del(9) (q13q) Karyotype in 3 of 17
metaphase cells analyzed.
Discussion
Complete or subtotal deletions of 9q have been associated with various somatic abnormalities and some solid tumors. Somatic abnormalities include facial dysmorphism, microcephaly and congenital heart defects [4-7]. Functional neurological abnormalities have also been described and include severe developmental delay, including motor and mental retardation as well as neuropsychiatric abnormalities. Sub-telomeric deletion at 9q34 can also lead to a characteristic syndrome with features including mid-facial hypoplasia, hypertelorism, anteverted nares, congenital heart disease, abnormal toes and developmental delays [6]. Other findings include hypotonia, microcephaly, and low set ears [8,9]. Most of these abnormalities are related to deletions or loss of function of the sub-telomere region of chromosome 9 (9q34.3) that range from less than 400kb to greater than 3Mb allelic loss. These anomalies are caused by loss or mutation of EHMT1 (Eu-HMTase1) gene that is responsible for the protein histone H3 Lys 9 (H3-K9) methyltransferase. H3-K9 histone has been shown to cause methylation and the subsequent loss of function of genes on the long arm of chromosome 9 [4,5].
Solid tumors have also been described following complete or partial 9q deletion. Gorlin Syndrome is an autosomal dominant disorder characterized by multiple basal cell carcinomas, medulloblastomas and ovarian fibromas. These malignancies are caused by null mutations or deletions rather than activating mutations. The gene NBCCS maps to chromosome 9q22, and allelic loss at this location is a common finding in these patients [8]. Deletion of 9q is known to lead to loss of important genes involved in the normal apoptotic process. Loss of certain genes located in the long arm of chromosome 9 such as TLE1 and TLE4 also lead to increased cell-cycle progression with increased expression of proliferative markers including Ki-67 and cyclin D1 [10,11]. Interstitial deletion of 9q is also a recurring abnormality in human myeloid hematologic malignancies. As early as 1971 Ward and Reinhard described the long- term outcomes in 72 patients with neutrophilic leukocytosis [12]. None of the cases described then progressed to any form of acute leukemia. However with a lack of molecular and genetic testing modalities, the etiology of this abnormality in the absence of any known secondary cause remained unclear. Interstitial deletions of 9q can still occur in approximately 2% of AML cases. This as a sole anomaly or together with t(8;21) [13] or other clonal chromosomal abnormalities [12]. It can also be seen in myelodysplastic and some myeloproliferative disorders. Deletion of 9q is sometimes associated with ALL as well. ABL1 deletion on chromosome 9 without the BCR-ABL1 fusion gene has been reported rarely in otherwise normal karyotype B-ALL [11].
The chronic leukocytosis in our patient is predominated by neutrophils. Common etiologies of chronic neutrophilia include infections, inflammation, medications, hemorrhage, splenectomy and hemolytic anemia. Other possible benign causes include physical and emotional stress, smoking and obesity. Leukocytosis secondary to inflammation follows tissue necrosis, infarction, from burns and arthritis [14]. Medications commonly associated with leukocytosis include corticosteroids, epinephrine, lithium and beta agonists. Splenectomy causes a transient leukocytosis that usually lasts for a few weeks. It is rare for it to last for several months. Physical or emotional factor related leukocytosis is called “stress leukocytosis” and may result from physical overexertion, seizures, anxiety, anesthesia, and epinephrine administration [14]. Obesity represents a state of chronic low-level inflammation and thus is recognized as a possible cause for reactive leukocytosis. Higher waist circumference indicates a large amount of abdominal visceral fat and this is known to promote inflammation [15]. Similarly smoking represents a state of chronic inflammation and is usually accompanied by moderate neutrophilia [16].
Conclusion
Isolated BCR-ABL negative partial 9q deletion can present as chronic neutrophilia. The bone marrow is morphologically normal and other molecular or cytogenetic abnormalities are absent. The pathophysiology appears to be related to loss of important genes involved in normal cellular apoptotic processes. The loss of certain genes located on the long arm of chromosome 9 such as TLE1 and TLE4 lead to increased cell-cycle progression with increased expression of proliferative markers including Ki-67 and cyclin D1. We suggest that after ruling out common secondary causes of chronic neutrophilia, loss of function mutations involving the long arm of chromosome 9 should be considered in BCR-ABL and JAK2 negative myeloproliferative states.
References
- Dallman PR (1977) Blood forming tissues. In: Rudolph A. ed. Pediatrics (16th edn), Appleton-century-Crofts, New York, USA, pp: 1100-1178.
- Kayashima T1, Yamaguchi K, Akiyoshi T, Nanimatsu H, Aragaki S, et al. (1993) Leukemoid reaction associated with diabetic ketoacidosis- with measurement of plasma granulocyte colony stimulating factors. Intern Med 32(11): 869-871.
- Juturi JV (1998) Severe leukocytosis with neutrophilia (leukemoid reaction) with alcoholic steatohepatitis. Am J Gastroenterol 93(6): 1013.
- Stewart DR, Kleefstra T (2007) The chromosome 9q sub-telomere deletion syndrome. Am J Med Genet Part C Semin Med Genet 145C: 383- 392.
- Yatsenko SA, Cheung SW, Scott DA (2005) Deletion 9q34.3 syndrome: genotype-phenotype correlations and an extended deletion in a patient with features of Opitz C trigonocephaly. J Med Genet 42: 328-335.
- Kannu P, Winship I, Aftimos S (2005) Further Case Report of a Child With a 9q34 Deletion and a Review of the Reported Cases. American Journal of Medical Genetics 133A: 219-221.
- Siggberg L, Peippo M, Marjatta Sipponen, Taina Miikkulainen , Keiko Shimojima , et al. (2011) 9q22 deletion-First Familial Case. Orphanet J Rare Dis 6: 45.
- Gailani MR, Siu VM, Yang-Feng T, Pressman CL, et al. (1996) Molecular analysis of chromosome 9q deletions in two Gorlin Synrome patients. Am J Hum Genet (59): 417-422.
- Tug e (2012) A patient with 9q subtelomeric deletion syndrome with additional findings. Genet Couns 23(4): 465-471.
- Dayyani F, Jianfeng W, Jing-Ruey J, Ahn EY, Tobey E, et al. (2008) Loss of TLE 1 and TLE 4 from the del(9q) commonly deleted region in AML cooperates with AML1-ETO to affect myeloid cell proliferation and survival. Blood 111(8): 4338-4347.
- Huh J, Mun Y (2012) ABL1 deletion without BCR/ABL1 rearrangement is originated from a large-sized 9q deletion. Ann Hematol 91: 1813-1815.
- Ward HN, Reinhard EH (1971) Chronic idiopathic Leucocytosis. Ann Int Med (75): 193-198.
- Molucca C, Vermaelen K, Kulling G, Michaux JL, Noens L, et al. (1984) Interstitial 9q-Deletions in Hematologic Malignancies. Cancer Genetics and Cytogenetics 12(4): 309-319.
- Abramson N, Melton B (2000) Leukocytosis: Basics of Clinical Assesment. Am Fam Physician 62(9): 2053-2060.
- Herishanu Y, Rogowski O, Polliack A (2006) Leukocytosis in obese individuals: possible link in patients with unexplained persistent neutrophilia. Eur J Hematol 76(6): 516-520.
- Dasanu C, Codreanu I (2012) Persistent polyclonal B-Cell lymphocytosis in chronic smokers: More than meets the eye. Conn Med 76(2): 69-72.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...