Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5945
Case Report(ISSN: 2638-5945) 
Case Report of Multiple Myeloma Presenting with Unique Skin Manifestation with Literature Review Volume 4 - Issue 2
Abdul Miah*
- Division of Medical Oncology, Department of Internal Medicine, The Ohio State University College of Medicine, USA
Received: January 16, 2021 Published: January 27, 2021
Corresponding author: Abdul R. Miah, Division of Medical Oncology, Department of Internal Medicine, The Ohio State University, 460 W 10th Ave, Columbus, OH 43210, USA
DOI: 10.32474/OAJOM.2021.04.000185
Abstract
Multiple myeloma (MM), although a rare disease, is the second most common hematologic malignancy. MM is associated with significant morbidity due to its end-organ destruction. It is a disease of the older population and advancements in the diagnosis, monitoring, and treatment of MM are of the utmost importance as the general population lives longer due to other improvements in health care. Herein, we present an interesting case of MM presenting with initially skin manifestation which unfortunately is associated with a poor prognosis due to a relationship indicating higher tumor burden. Despite being on multiple treatment, our patient continued to progress with her disease.
Introduction
Multiple myeloma (MM) accounts for approximately 2% of all malignancies and 13% of hematological malignancies worldwide [1]. MM is the second most common hematologic malignancy [1] and it is a neoplasm of clonal plasma cells which originate from the B-cell lineage and develop after lineage commitment in the bone marrow of progenitor cells [9]. Cutaneous involvement in patients with multiple myeloma is rare and it usually represents of a poor prognosis. Cutaneous involvement indicates an increased tumor burden. The most common cause of cutaneous involvement is due to direct extension from underlying bone lesions of MM or solitary plasmacytoma of bone [2]. It is very rare to have primary cutaneous plasmacytoma [3].
Case Presentation
We present the case of a friendly Caucasian American 70-yearold
female with no significant PMH who initially presented to
Ruby Memorial Emergency Department in November 2015 with
persistent back and left side pain. At that time, she began to
notice weakness and some numbness in lower extremities and
along with the increasing low back pain decided to seek medical
attention and presented to the hospital. On admission, CT thoracic
spine (11/16/15) revealed a T5 wedge compression deformity.
CT lumbar spine revealed degenerative changes with diffuse
osteoporosis. MRI thoracic spine (11/17/15) revealed a T5 burst
fracture with retropulsion, focal kyphotic deformity, severe central
canal stenosis, possible pathologic fracture deformity, And possible
cord contusion at T5. CBC on admission revealed WBC 5.1 with
normal differential, Hgb 11.5 with MCV 105.3 and platelet count
170,000. BMP normal, creatinine 0.75, corrected calcium 10.2.
Subsequently, she underwent a posterior spinal fusion T3-T8,
left costotransversectomy with left pedulectomy and subtotal
corpectomy (excision of tumor) via transpedicular approach with
placement of anterior cage, T5 laminectomy, and T6-7 partial
laminectomy. Pathology revealed a tumor composed of sheets of
atypical plasmacytoid cells positive with immunostain for CD138
and negative for pancytokeratin, CD20, and CD3. Insufficient tissue
remained for kappa and lambda ISH. The finding was consistent
with plasma cell neoplasm. SPEP revealed an M spike seen in the
gamma, consistent with monoclonal gammopathy. Random UPEP/
IFE revealed a monoclonal lambda protein without detectable
associated IgG, IgA or IgM heavy chain. In December, SPEP/IFE
revealed an IgG Lambda monoclonal protein (5.95 g/dL). Free
light chain ratio 0.013 (Kappa 0.80 mg/dL, Lambda 60.50 mg/dL).
Quantitative IgG 5950 g/dL. Hgb 9.4 with MCV 98.5. Creatinine 0.62. Corrected serum calcium 11.2. Albumin 2.0. Beta 2 microglobulin
4.25. Skeletal survey reveals diffuse osteolytic process with multiple
calvarial, vertebral body and rib lesions identified. T3-T8 posterior
spinal fusion for pathological T5 compression fracture noted.
She had a bone marrow biopsy/aspirate which revealed 47%
lambda restricted plasma cells, with marked atypia including
a plasmablastic morphology. Preliminary FISH results indicate
multiple trisomies, 1q21 gain, del (13q14) and a non-standard
IGH rearrangement-t(8;14). Hyperdiploidy detected. She was
diagnosed with IgG Lambda Multiple Myeloma. ISS Stage II, DS
IIIA. Soon thereafter, she was started on a bortezomib-based
triplet regimen - bortezomib, cyclophosphamide, dexamethasone
(CyBorD). She completed 4 cycles. In April 2016, SPEP/IFE revealed
an IgG Lambda monoclonal protein - not quantified. Quantitative
immunoglobulins: IgG 1013 mg/dL. Free light chain assay
revealed a ratio of 1.169 (kappa 2.98 mg/dL, Lambda 2.55 mg/
dL). CBC normal. Hgb 13.2. Serum creatinine 0.59. Serum calcium
9.7 (corrected). Serum albumin 3.0 g/dL. Beta 2 microglobulin
2.65 mcg/mL. Skeletal survey demonstrated new evidence of L5
pathological compression fracture. A repeat bone marrow biopsy
revealed a variably cellular marrow (10-50%). Maturing trilineage
hematopoiesis noted. No atypical plasma cell infiltrate (<5% by
CD138 ICH). Cytogenetics normal. Unable to run FISH. At the end of
the month, her regimen switched to Lenalidimide/Dexamethasone
and she completed 4 cycles. In August, she began maintenance
therapy with Lenalidomide at 10 mg daily. She then presented with
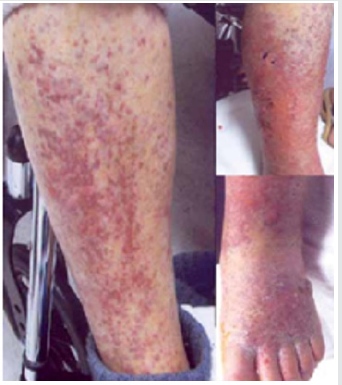
multiple skin papule skin lesions (Figure 1) mostly on her gluteal
region. In January 2017, she had a left gluteal skin biopsy consistent
with plasma cell neoplasm with high grade features (plasmablastic
differentation) In February 2017, SPEP/IFE revealed a IgG Kappa
monoclonal protein (not quantified). IgG level 2303 mg/dL. Free
light chain assay reveals a ratio of 1.31 (Kappa 12.63 mg/dL, Lambda
9.62 mg/dL). She completed radiation therapy to left gluteal skin
- received 30 Gy/15 fractions. SPEP/IFE revealed a IgG Kappa
monoclonal protein (not quantified). IgG level 2357 mg/dL. Free
light chain assay reveals a ratio of 1.47 (Kappa 12.20 mg/dL, Lambda
8.32 mg/dL). In March 2017, she was started on Pomalidomide/
Dex. Unfortuatnely she was found to have progression of her
disease noted. She was then started on Daratumumab which she
could not tolerate and was then started on Carfilzomib/Dex. She
has received multiple radiation therapy to her left gluteal region,
mid back and left upper lip with significant resolution in all areas.
In February 2017, she was admitted to Ruby with worsening pain
and lesions located in her lower extremities and edema (Figure 1).
Peripheral duplex was obtained and blood cultures to rules out
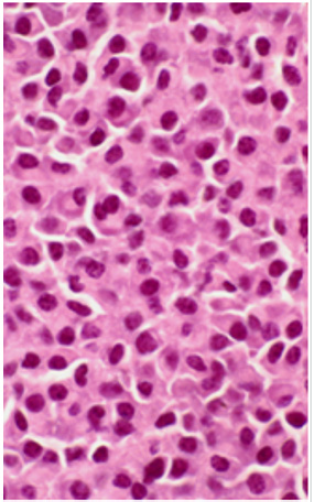
a venous clot and infection respectively. A biopsy was obtained
(Figure 2) which showed cutaneous involvement of her multiple
myeloma. Radiation Oncology was consulted, and she was started
on palliative radiation for 5days which initially improved her pain
and swelling. Unfortunately, her lesions continue to arise with
intermittent, short lived responses to systemic therapy. Her lesions
now too widespread for continued radiation therapy. She noticed
lesion in her scalp. She continues to follow up in clinic where she
was started on single agent Doxil 40 mg/m2 every 4 weeks. Sadly,
she continues have progressive cutaneous disease around the flank,
abdomen, and bilateral thighs.
Discussion
We present an interesting case of cutaneous involvement of multiple myeloma where although the patient has been on multiple therapies continues to progress in her disease. Her case is interesting from other presentations in that they don’t usually do not describe the course of regimen used in treatment. The standard of care of cutaneous involvement revolves controlling the origin of the disease. One specific treatment that we tried was localized radiation. There is not much data or past literature discussing the use of radiation or its efficacy. Unfortunately for our case, the treatment only helped briefly. Most common involvement for MM is soft tissue involvement of the upper airway and oral cavity. They usually consist of firm, erythematous, nontender nodules involving the neck, ears, shoulders, axillae, chest, abdomen, and dorsum of the hands [11]. The first reported case of skin involvement in a person with MM was presented by Bruno Block in 1910. He described a patient who had small reddish macules that evolved into brown reddish papules and nodules with scale crusts. Histologically these lesions showed epidermal necrosis. He eventually had disease in pleura, stomach, and heart and passed away two years later [3]. A review of literature reveals that there are over 100 described cases. The age ranges from 36 to 81 with a median of 60 years old. Numeric date was available for 87 cases and 63 of them were male and 24 were female [4]. Cutaneous involvement of MM may appear in area of the skin, but it has been reported most commonly on the trunk and abdomen. Skin lesions is commonly described as papules or nodules that measure 1-5 cm in diameter with firm consistency, smooth surface, and a red or violaceous color [5]. Some authors reported that cutaneous involvement of MM only occurs when the tumor mas burden is over 2-3 kg [6]. Cutaneous involvement in patient with MM and extramedullary plasmacytoma generally appears late during the disease. On average, death occurred within 12 months after the diagnosis. Autopsy of these patients reveal extensive plasmacytic infiltration of multiple organs [7].
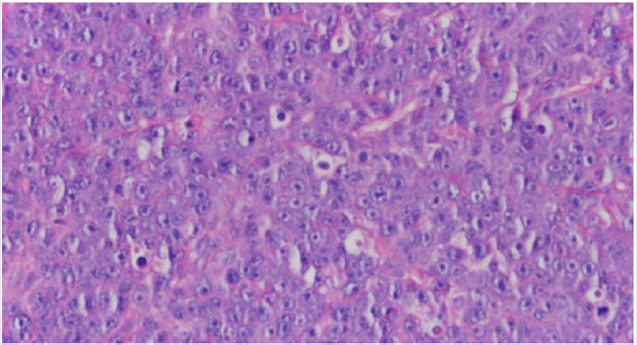
A review of the cases of MM involving the skin revealed that 40 cases were IgG, 21 cases were IgA, and 9 cases were IgD. The risk of cutaneous involvement by MM is not associated with a particular class of myeloma immunoglobins. Histopathologically, the lesions of MM involving the skin show 2 patterns: nodular and diffuse interstitial [6]. (Figure 2, 3). The worldwide incidence of myeloma is 86,000 cases annually. Mortality rate in MM is high with a median survival of 50-55 months and 63,000 deaths being reported worldwide each year [8]. Significant advances have been made in understanding multiple myeloma (MM) and its precursor diseases. These advances include the gain in knowledge in the underlying pathophysiology, Food and Drug Administration (FDA) approvals of novel therapies with meaningful efficacy and the science in underlying disparities in patients with MM [9].
Author would like to thank Dr. Mortazavi and Arham Miah for their support.
Figure 3: Diffuse Pattern.
Parametrium to be excised
x—x: as necessary from the caudal edge of the cervix.
References
- Kazandjian D (2016) Multiple myeloma epidemiology and survival: A unique malignancy. Semin Oncol 43(6): 676-681.
- Requena L, Kutzner H, Palmedo G, Calonje E, Requena C, et al. (2003) Cutaneous Involvement in Multiple Myeloma: A Clinicopathologic, Immunohistochemical, and Cytogenetic Study of 8 Cases. Archives of Dermatology 139(4): 475-486.
- Naymagon L, Abdul Hay M (2019) Primary extramedullary plasmacytoma with diffuse lymph node involvement: a case report and review of the literature. J Med Case Rep 13(1): pp. 153.
- Tuchman SA, Shapiro GR, Ershler WB, Badros A, Cohen HJ, et al. (2015) Multiple myeloma in the very old: an IASIA conference report. J Natl Cancer Inst. 106(5): dju067.
- Minni KA, Karuvelan TG, Chellam J, Krishanaswamy M (2015) Multiple Myeloma with Cutaneous Deposits as Rare Lymphoplasmacytoid Cells. Indian J Dermatol 60(4): pp. 424.
- Requena L, Kutzner H, Palmedo G, Calonje E, Requena C, et al. (2003) Cutaneous involvement in multiple myeloma:a clinicopathologic, immunohistochemical, and cytogenetic study of 8 cases. Arch Dermatol 139(4): 475-486.
- Oshima K, Kanda Y, Nannya Y, Kaneko M, Hamaki T, et al. (2001) Clinical and pathologic findings in 52 consecutively autopsied cases with multiple myeloma. Am J Hematol 67(1):1-5.
- Cowan AJ, Allen C, Barac A, Basaleem H, Bensenor I, et al. (2018) Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol 4(9): 1221-1227.
- Kazandjian D, Mailankody S, Korde N, Landgren O (2014) Smoldering multiple myeloma: pathophysiologic insights, novel diagnostics, clinical risk models, and treatment strategies. Clinical advances in hematology & oncology 12(9): 578-587.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...