Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5945
Editorial(ISSN: 2638-5945) 
What is beyond the Nivolumab Monotherapy approval for advanced Hepatocellular Carcinoma? Volume 1 - Issue 2
Luis Mendoza*
- Senior Medical Advisor, IQVA, Europe
Received: March 21, 2018; Published: March 26, 2018
Corresponding author: Luis Mendoza, Senior Medical Advisor Medical Strategy & Science, Oncology-Hematology, Therapeutic Science & Strategy Unit, IQVA
DOI: 10.32474/OAJOM.2018.01.000109
Keywords: Hepatocellular carcinoma; Immune checkpoint inhibitors; Nivolumab; FDA
Editorial
With an estimated 500,000 new cases per year, hepatocellular carcinoma (HCC) represents the third leading cause of cancer death worldwide. The incidence is rising in the west, largely due to an increasing incidence of hepatitis C virus infection [1]. The majority of HCC patients are diagnosed with disease too advanced for curative treatment. Only liver resection and liver transplantation are considered curative, with poor efficiency of other modalities such as radiofrequency ablation (RFA) and transarterial chemoembolization (TACE), although this may provide a modest prolongation in survival; however, the relapse in the majority of these patients is inevitable [2]. An array of translational research and pilot clinical trials have revealed that adoptive immunotherapy's are safe by patients with HCC, but they lack efficacy [3]. Now, we are in the new era of immunotherapy's such as immune checkpoint inhibitors and CAR-T strategies, which would bring benefit to the HCC patients.
On September 22, 2017, the Food and Drug Administration granted accelerated approval to nivolumab (OPDIVO, Bristol- Myers Squibb Co.) for the treatment of HCC in patients who have been previously treated with sorafenib. The approval was based on a 154-patient subgroup of CHECKMATE-040 (NCT 01658878), a multicenter, open-label trial conducted in patients with HCC and Child-Pugh. A cirrhosis who progressed on or were intolerant to sorafenib. Patients received nivolumab 3 mg/kg by intravenous infusion every two weeks. The confirmed overall response rate, as assessed by blinded independent central review using RECIST 1.1, was 14.3% (95% CI: 9.2, 20.8), with three complete responses and 19 partial responses. The response duration ranged from 3.2 to 38.2+ months; 91% of responders had responses lasting six months or longer and 55% had responses lasting 12 months or longer. Adverse reactions occurring in patients with HCC in CHECKMATE-040 were similar to those previously reported in product labelling, with the exception of a higher incidence of elevations in transaminases and bilirubin levels [4].
There are other immune checkpoint inhibitors that are being tested as monotherapy for efficacy and safety in HCC. Nivolumab was the first approved, but others will follow, as it has occurred in other malignancies. There is a bunch of possibilities for the treatment strategy using immune checkpoint inhibitors. Future directions point to various stages of HCC treatment, such as neo adjuvants and adjuvants after resection and ablation, combination therapy with transcatheter arterial chemoembolization, first-and second-line treatments, and all sorts of combinations with other immunotherapies, targeted molecules and novel therapies.
In the table annexed to this editorial you will find a list of ongoing clinical trials combining the immune checkpoint inhibitors with other therapies. At the top of the Table 1 are listed the trials with simultaneous blockage with anti-PD-1/PD-L1 and anti- CTLA-4 antibodies, which are expected to be promising regimens in HCC immunotherapy. The high efficacy of the combination therapy was demonstrated in malignant melanoma [5]. Simultaneous inhibition of the B7-CTLA-4 pathway by an anti-CTLA-4 antibody may increase the number of activated CD8+ T cells in lymph nodes, followed by an increase in the number of activated CD8+ T cells infiltrating the tumour tissues, thereby enhancing the antitumor effects. Their combination with molecular targeted agents (e.g., sorafenib or axitinb) also appears promising.
Table 1: Summary of ongoing trials with immune checkpoint inhibitors in HCC.
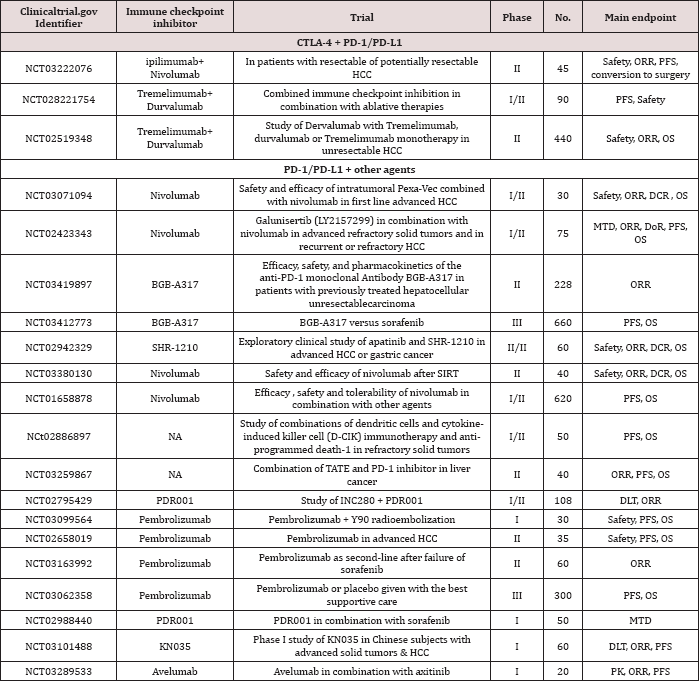
In particular, the approach combining an immune checkpoint inhibitor with an existing loco regional therapy for HCC is currently under evaluation. TACE or RFA is expected to enhance the effects of immunotherapy by inducing local inflammation, releasing gneoantigens that activate antigen presentation and immune system activation. The results of the combination therapy with anti- CTLA-4 antibody and loco regional therapy in advanced HCC have recently been published [6]. The NCT01853618 study evaluated the efficacy of adjuvant therapy with tremelimumab (anti-CTLA-4 antibody) after RFA or TACE in several, but not all, HCC nodules, with favourable outcomes, including a partial response rate of 26%, time to tumor progression of 7.4 months, and overall survival of 12.3 months.
The results of trials of the immune checkpoint inhibitor- combined strategies are awaited with high expectations by the medical community.
References
- El-Serag HB, Mason AC (1999) Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 340: 745-750.
- Bruix J, Sala M, Llovet JM (2004) Chemoembolization for hepatocellular carcinoma. Gastroenterology 127(5(1)): S179-S188.
- Yu-Peng H, Zi-Duo L, Pankaj P, Qi Z (2015) Immunotherapy for hepatocellular carcinoma: From basic research to clinical use. World J Hepatol 7(7): 980-992.
- FDA, US Food and drog Administartion, USA.
- Larkin J, Chiarion-Sileni V, Gonzalez R, Jean Jacques Grob, C Lance Cowey, et al. (2015) Combined nivolumaband ipilimumab or monotherapy in untreated melanoma 373: 23-34.
- Duffy AG, Ulahannan SV, Makorova-Rusher O, Wedemeyer H, Pratt D, et al. (2017) Tremelimumabin combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol 66: 545-551.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...













