
Lupine Publishers Group
Lupine Publishers
Menu
Case Report(ISSN: 2641-6875) 
Sepsis Due to Co-Infection with Human Papillomavirus and Acinetobacter Schindleri after Surgery for Uterine Fibroids Volume 2 - Issue 4
Sanaz Rajabi Khamseh* and Abdolrazagh Danesh Shahraki
- Department of Agronomy, Faculty of Agriculture, Shahrekord University, Iran
Received:October 25, 2021; Published:November 24, 2021
*Corresponding author: Falin Chen, Clinical Microbiology Laboratory, Fujian Provincial Hospital, China
DOI: 10.32474/CTBM.2021.02.000142
Keywords: Azotobacter; Bacillus; Drought; Germination; Oilseed
Case
A 33-year-old woman underwent a physical examination at the
Department of Gynecology and was found to have uterine fibroids.
She was admitted for laparoscopic removal of the uterine fibroids
under general anesthesia. During the operation, there was a small
amount of dark red ascites in the pelvic cavity, a part of the intestine
was densely adhered to the right pelvic wall, and the uterus was
enlarged to the size of 10 weeks gestation, with an uneven surface.
There was a fibroid tumor measuring approximately 7 × 8 × 8 cm
on the posterior wall of the uterus, which was hard with clear
boundaries. Two vesicular clear-fluid cysts with a diameter of 1-1.5
cm were seen on each fallopian tube. Due to the large posterior
wall myoma and difficulty in suturing, the patient was considered
barren; hence, abdominal myomectomy and pelvic adhesiolysis
were selected, followed by indwelling catheterization. On the first
day after surgery, the patient felt cold and was shivering, with a
temperature of 38.5°C and lower abdominal pain. There was no
sign of infection in the abdominal incision. When the indwelling
catheter was removed, there was no urethral inflammation
observed and the patient was able to urinate without difficulty.
Blood was immediately drawn for culture and biochemical tests,
which revealed low levels of sodium, calcium, and magnesium, and
abnormal coagulation function. The level of original calcitonin was
normal. The blood culture was positive after 15.3 hours in a nutrient
solution for gram-negative bacilli. A diagnosis of sepsis was made
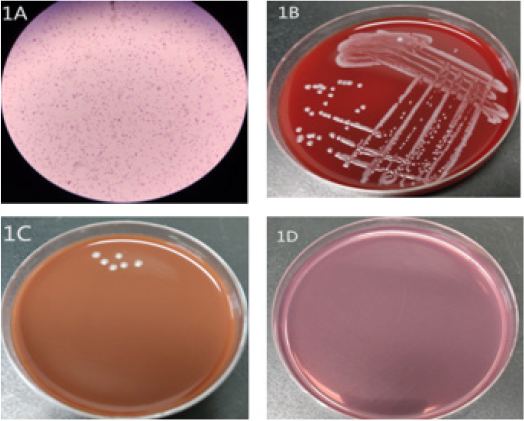
based on the clinical features and laboratory values. Gram staining
revealed short Gram-negative bacilli (Figure 1A). The blood culture
plate showed gray and white colonies with good growth (Figure
1B). The chocolate culture plate showed poor growth (Figure 1C),
while the MacConkey plate did not show any organism growth.
(Figure 1D).
(Figure1A) Gram staining (1,000×) of Acinetobacter schindleri
showing short, straight Gram-negative bacilli. (Figure1B) Good
growth of gray and white colonies after culture on Columbia agar
with 5% sheep’s blood after 48 h of incubation. (Figure1C) Poor
growth of gray and white colonies after culture on chocolate agar
after 48 h of incubation. (Figure1D) No growth after culture on
MacConkey agar. As bacterial identification was not possible with
the VITEK II automatic bacterial analyzer (BioMerieux, Craponne,
France), the VITEK-MS mass spectrometer (BioMerieux) was used
to identify the bacteria as Acinetobacter schindleri. The results of
the drug sensitivity testing for A. schindleri, based on the American
Society for Clinical Laboratory Standardization (2017), were
as follows: amoxicillin/papaulic acid 2 μg/ml, amikacin 2 μg/
ml, ciprofloxacin 0.25 μg/ml, cefoperazone/sulbactam 34 μg/
ml, cefepime 1 μg/ml, gentamicin 1 μg/ml, imipenem 1 μg/ml,
levofloxacin 0.25 μg/ml, minocycline 28 μg/ml, sulfamethoxazole 20 μg/ml, tigecycline 0.5 μg/ml, tobramycin 1 μg/ml, and piperacillin/
tazobactam 4 μg/ml. A. schindleri was resistant to amoxicillin/
papaulic acid and sensitive to all others. In addition, 16SrRNA was
used to identify the bacteria, and the sequence comparison analysis
results were A. schindleri (NR025412.1) 99.22% and Acinetobacter
haemolyticus (NR117622.1) 97.59%. The patient responded
well to cefoperazone/sulbactam and metronidazole antibiotic
treatment. After 5 days of treatment, the patient had normal body
temperature, improved symptoms, and negative blood cultures; she
was discharged two weeks later.
Figure 1: (1A) Gram staining (1,000×) of Acinetobacter schindleri showing short, straight Gram-negative bacilli. (1B) Good growth of gray and white colonies after culture on Columbia agar with 5% sheep’s blood after 48 h of incubation. (1C) Poor growth of gray and white colonies after culture on chocolate agar after 48 h of incubation. (1D) No growth after culture on MacConkey agar.
Discussion
A. schindleri was first reported in [1]. A. schindleri is a nonmotile,
aerobic, gram-negative bacterium that can grow on dry
surfaces for a long time [2] and exist in a wide range of natural
environments, such as soil and water, as well as in hospitals [3].
The strains can be isolated from body surfaces of patients, and their
presence in patient specimens (vagina, cervix, throat, nasal cavity,
or urine) is usually clinically insignificant [1]. In 2012, a case was
reported of A. schindleri carrying bla-NDM-1 found in the groin
area of a 22-year-old man who had suffered an explosion injury
during the war in Afghanistan; however, the clinical significance of
this finding was not mentioned [4]. Studies have found that 80% of
cases of catheter-related bacteremia show growth of A. schindleri
in blood cultures; the endovascular device acts as a portal for A.
schindleri blood infection [2]. A. schindleri is a gram-negative
opportunistic pathogen that is prevalent in intensive care units and
affects immunocompromised patients, thus leading to nosocomial
infections and global outbreaks [5]. Compared with laparoscopic
hysterectomy, traditional open myomectomy can reduce the blood
supply to the anastomotic branches of the uterine and ovarian
arteries causing poor local uterine blood circulation and increased
vascular permeability, which can cause the bacteria to colonize
the uterus and invade the bloodstream [6]. Due to the complex
anatomical structure of the pelvic cavity and the large surgical
wound, open surgery can easily cause body injury and immune
suppression; the surgical process also increases the probability
of opportunistic pathogen invasion. The patient had a history of
human papillomavirus infection, a high-risk factor for cervical
cancer.
The occurrence of tumor development is closely related to
the body’s immune function; when there is progressive tumor
growth, immune function is restrained. Therefore, in cases such
as large trauma surgery, severe postoperative complications in
patients with all the above factors can cause low immunity, leading
to opportunistic pathogens like A. schindleri infection. The only
microorganism isolated from the blood culture of our patient was A. schindleri, indicating that it was the pathogen causing the
patient’s postoperative sepsis. Blood culture remains the gold
standard for the diagnosis of sepsis. Since 1986, the classification
of Acinetobacter has undergone extensive revisions, with the
discovery of a wide variety of species; however, studies have found
that it is impossible to identify specific species of Acinetobacter
by phenotype [2]. A. schindleri can be identified by a VITEK - MS
spectrometer. However, clinical microbiology laboratories should
be aware that while A. schindleri grows well in blood culture
plates, it grows poorly in chocolate culture plates, and not at all in
MacConkey plates. The risk factors for Acitenobacter infection are
low immunity, underlying health conditions, and a history of human
papillomavirus infection. Clinical attention should be paid to the
implementation of active perioperative nursing care, strict aseptic
operation, and reduction of postoperative nosocomial infections.
References
- Nemec A, Dijkshoorn L, Jezek P (2000) Recognition of Two Novel Phenons of the Genus Acinetobacter Among Non-Glucose-Acidifying Isolates from Human Specimens. Journal of Clinical Microbiology 38(11): 3937-3941.
- Dortet L, Legrand P, Soussy CJ, Cattoir V (2006) Bacterial Identification, Clinical Significance, and Antimicrobial Susceptibilities of Acinetobacter Ursingii and Acinetobacter Schindleri, Two Frequently Misidentified Opportunistic Pathogens. Journal of Clinical Microbiology 44(12): 4471-4478.
- Bergogne-Bérézin E, Towner KJ (1996) Acinetobacter spp. as Nosocomial Pathogens: Microbiological, Clinical, and Epidemiological Features. Clinical Microbiology Reviews 9(2): 148-165.
- McGann P, Milillo M, Clifford RJ, Snesrud E, Stevenson L, et al. (2013) Detection of New Delhi Metallo-β-lactamase (encoded by blaNDM-1) in Acinetobacter Schindleri During Routine Surveillance. Journal of Clinical Microbiology 51(6): 1942-1944.
- Forster DH, Daschner FD (1998) Acinetobacter species as Nosocomial Pathogens. European Journal of Clinical Microbiology & Infectious Diseases: Official Publication of the European Society of Clinical Microbiology 17(2): 73-77.
- Tian Y, Chen J (2021) The Effects of La paroscopic Myomectomy and Open Surgery on Uterine Myoma Patients Postoperative Immuno-Inflammatory Responses Endocrine Statuses and Prognoses: A Comparative Study. American Journal of Translational Researc 13(8): 9671-9678.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...





