Lupine Publishers Group
Lupine Publishers
Menu
Mini Review(ISSN: 2641-6875) 
Microbial Remediation of Dichloro-Diphenyl- Trichloroethane (DDT) Volume 2 - Issue 2
Girma Ebsa*, Tesfaye Alemu and Birhanu Gizaw
- Department of Microbial, Cellular and Molecular Biology, Addis Ababa University, Ethiopia
Received:May 05, 2021; Published:May 25, 2021
*Corresponding author:Girma Ebsa, Department of Microbial, Cellular and Molecular Biology, Addis Ababa University, Addis Ababa, Ethiopia
DOI: 10.32474/CTBM.2021.02.000134
Abstract
The insecticide had been intensively applied for agricultural pest control since 1940. It was disqualified because it persists in the environment, accumulates in fatty tissues, and can cause bad health effects on wildlife and human being. The organochlorine (DDT) has been programmed under the Stockholm Convention to protect human health and the environment from Persistent Organic Pollutants (POPs). Biodegradation is carried out by microorganisms (bacteria and fungi) that naturally live in the environment. Bacteria and fungi have very diverse metabolisms, and they use a wide variety of food and energy sources and perform many important functions by decomposition. Complete biodegradation of DDT involves the oxidation of parent compound to form carbon dioxide and water and provides both carbon and energy for the growth and reproduction of microbes. Each degradation step is catalyzed by specific enzyme produced by a degrading cell or enzyme found external to the cell. Degradation of insecticide by enzyme will stop at any step if an appropriate enzyme is not present. Effects of DDT on human health and the environment depend on the dose of DDT and the timespan and frequency of exposure. It effects also depend on the health of a person and certain environmental factors. DDE and DDT can pass to the fetus in pregnant women. Both chemicals are found in breast milk, resulting in exposure to nursing infants. Microbes can be screened out from soil and wastewater as an effective tool for biodegradation of toxic organic chemicals. Phanerochaete and related fungi that have the ability to attack wood possess a powerful extracellular enzyme that, acts on a broad array of organic compounds.
Introduction
Ethiopia is a country of more than 1.1 million square kilometers, located in the Horn of Africa between approximately 40 and 150 north latitude and 320 and 490 east longitude. Ethiopia has rich in biodiversity of animals, plants and microorganisms where overuse of pesticides has adverse effects on the loss of natural recourses. The Ethiopian government identified the impact of these measures in preparation of the Conservation Strategy of Ethiopia (1997) and becoming a signatory in 1992 and ratifying the Convention on Biological Diversity (CBD) in 1994 (Et-NBSAP, 2015). In Ethiopia and worldwide, farmers used DDT on a variety of food crops. DDT is a practicable insecticide in indoor residual spraying owing to its effectiveness in well supervised spray operation and high excito-repellency factor. Although DDT is very effective in killing or repelling mosquitoes its use has been severely reduced and restricted to indoor residual spraying, due to its persistence in the environment and ability to bioconcentrate in the food chain [1,2]. DDT was so broadly used because of effective, relatively inexpensive to manufacture, and lasts a long time in the environment [3]. It was disqualified because it persists in the environment, accumulates in fatty tissues, and can cause bad health effects on wildlife [4]. Resistance occurs in some insects (like the house fly) that develop the ability to quickly metabolize the DDT (WHO, 1979). DDT affects the nervous system by interfering with normal nerve impulses [3]. The organochlorine (DDT) has been programmed under the Stockholm Convention to protect human health and the environment from Persistent Organic Pollutants (POPs). The Convention aims to decrease and ultimately eradicate DDT but recognizes the acceptable production and use of DDT for disease vector control. DDT is one of the insecticides recommended by WHO for indoor residual spraying for malaria control (ATSDR, 2002). The Stockholm Convention has been established for DDT register in which countries are appreciated to report their intention to produce or use DDT. The continued need for DDT disease vector control, which is subject to evaluation by the conference of the parties during its regular meetings held every 2 years, was confirmed in 2015. Changes in DDT levels in humans and the environment have been reported elsewhere. As this is the first effectiveness evaluation cycle, it will provide a baseline for future evaluations. In addition, the time period covered by the evaluation allows for the study of trends (CECP, 2006). Biodegradation is carried out by microorganisms (bacteria and fungi) that naturally live in the environment. Bacteria and fungi have very diverse metabolisms: they use a wide variety of food and energy sources and perform many important functions. One especially important function is decomposition. Decomposers are bacteria and fungi that can break down organic matter and in doing so recycle nutrients [5]. There have been several reports on the isolation and trait characterization of microbial communities that can perform functionally better in combination with the existing rhizosphere bacteria, beneficial mycorrhizal fungi and biological control agents [6]. Complete biodegradation of DDT involves the oxidation of parent compound to form carbon dioxide and water and provides both carbon and energy for the growth and reproduction of microbes. Each degradation step is catalyzed by specific enzyme produced by a degrading cell or enzyme found external to the cell. Degradation of insecticide by enzyme will stop at any step if an appropriate enzyme is not present. Absence of an appropriate enzyme is one of the common reasons for persistence of any pesticide. If an appropriate microorganism is absent in soil or if biodegrading microbial population has been reduced due to toxicity of pesticide in that case a specific microorganism can be added or introduced in soil to enhance the activity of the existing population [7].
Historical Application and Utilization Of DDT
In U.S further testing of the insecticide was conducted at department of agriculture’s laboratory in Orlando, Florida, in 1942 and 1943. After the discovery of the insecticidal properties of DDT in1939, the tests confirmed the practical value of DDT in disease vector control. DDT was firstly used by the military in WW II to control malaria, typhus, body lice, and bubonic infection (WHO, 1979). Malaria cases fell from 400,000 in 1946 to almost none in 1950 [8] and it was first synthesized by Zeidler in 1874; after that, in 1939 Paul Muller discovered its insecticidal properties [9]. DDT was first manufactured in 1943 [10]. DDT was a useful insecticide chemical until it was forbidden in most industrialized countries since the late 1970s.The insecticide had been intensively applied for agricultural pest control since 1940 [11]. In Ethiopia chemical pesticide use was historically low, but agricultural developments increased food production and expansion in floriculture industry have resulted in higher consumption of chemical pesticides (Et- NBSAP, 2015). Pesticide use in Africa accounts for only 2-4% of the global pesticide market of US $31 billion [12]. Organochlorines are chemical compounds which contain hydrogen, carbon, chlorine, and, sometimes, other atoms. DDT is an organochlorine insecticide. It was commonly used for insect control in the United States until it was canceled in 1972 by the United States Environmental Protection Agency (EPA). Even if it was banned; DDT is still used in some African countries including Ethiopia, where malaria vector control is significant. The use of DDT resulted in a successful elimination of the malaria vector [13]. The impact of DDT on mosquito longevity resulted in successful early operations against the malaria vector and calls for the global eradication of malaria followed [14]. The primary effect of DDT is its excito-repellency, avoiding entry into or driving mosquitoes out of sprayed houses and reducing transmission with lower feeding rates and shorter resting periods [15]. In 1955, the World Health Organization (WHO) launched the global malaria eradication campaign based on the periodic use of IRS with DDT for 3-5 years to interrupt malaria transmission (IDAB, 1956). This time-limited attack phase would have been followed by active case detection and surveillance to prevent disease propagation (IDAB, 1956). The eradication period ended in 1969, and the eradication strategy was replaced by a longer-term disease control strategy as part of the growing primary healthcare movement of the 1970s [14].
Pesticide Use and Impacts in Ethiopia
The Ethiopia environmental policy states the sound management and use of natural, human-made and cultural resources and the environment as a whole “to get better and increase the health and quality of life of all Ethiopians and to encourage sustainable social and economic development and to meet the needs of the present generation without compromising the ability of future generations to meet their own needs. The average crop loss due to pests was estimated between 30 and 40% annually [16]. The enhancement of Ethiopian agricultural development policies has resulted the use of inorganic fertilizers and chemical pesticides in order to increase agricultural productivity [17]. Chemical pesticides were launched in Ethiopia since 1960s for the development of commercial farms [18]. Currently, the Ethiopian government initiated to make the country food self-sufficient and increase agricultural export commodities like coffee, flowers and vegetables, to exploit the diversity by the use of agricultural growth. Pesticide sale supporting groups makes the farmers to believe that pesticides are the only way to avoid crop losses [16]. Ethiopia imports more than 3800 tons of pesticides annually and the country also gets pesticides by donations. The pesticides imported about are: 72% insecticides, 25% are herbicides, 2.6% are fungicides and 1.3% is other product such as rodenticides and disinfectants [16]. The country’s legalization for pesticide registration and a monitoring system were introduced in the late 1990’s. Even though there is legislation governing pesticide registration, clear guidelines on the importation, testing, and use of pesticides have not been effectively enforced. Therefore, it is common to find restricted or banned pesticides widely used in Ethiopia. There is also poor application practice without using Personal Protective Equipment’s (PPEs). This results in unprotected spreading of pesticides into the environment, and which causes human exposure to pesticides. Some bad practices of exposure and unprotected spray operators are shown in Figure 1 [19]. There is an enormous use of pesticides on large scale farms and less use of pesticides in small scale farming in Ethiopia [19]. The government extension services encourage the use of pesticides to improve productivity and to reduce food insecurity for the Ethiopian people living from small scale farming (ESAI, 2006). Besides the agricultural use, insecticides have been used for public health to control vector borne diseases such as malaria. DDT is the first line insecticide used for Indoor residue Spray (IRS), because it is the cheapest, relatively safe, and effective under close monitoring & long residual effect greater than 6 months, compared to all other chemicals as insecticide for public health use. Indoor Residual Spraying (IRS) is used to prevent malaria in epidemic prone areas [20]. For the last 40 years, Ethiopia has primarily used DDT for its IRS operation program [21]. The country applies approximately 400 metric tons of the active ingredients DDT per year [22]. Nowadays, the use of DDT for malaria vector control is phased out due to the resistance development by the malaria vector and replaced by effective insecticides such as primiphos-methyl and propoxur [23]. DDT were used widely in agriculture and for public health purposes, became a global concern because of their persistence, bioaccumulation potential and health effects to human and non-target organisms [24].
Figure 1: Outdoor and Indoor or Green house. B) Pesticide Application in Ethiopia. Human Exposure and Consumer Risk Assessment to Pesticide Use in Ethiopia.

Ethiopian mechanized agriculture considers pesticides as a best choice for improving agricultural productivity and quality of life in general. However, evidence in figure one of the last few decades have shown that the used pesticides can be detrimental to human health and the ecosystem [25]. Some of the reasons for these problems are illiteracy of the majority of the peasants, inadequate training, lack of awareness, lack of appropriate and timely information about the proper use and management of pesticides, inappropriate use of Personal Protective Equipment (PPE), wrong concept that pesticides are the best solution to pest control, and poor guidance about the safe use and handling. Inappropriate use of pesticides: wrong combination of different types of pesticides, use of pesticides for unplanned purposes, and utilization of empty pesticide containers for domestic purposes. Weak law enforcement: late issuance of regulations and guidelines, inadequate implementation of the issued regulations, weak monitoring or follow-up activities to control pesticide usage. Disposal problems: there is no disposal facility in Africa; particularly in Ethiopia due to the high cost of disposal and the cost of appropriate destruction of obsolete pesticides is too high. Due to these problems, the contamination of the different environmental compartments (water, air, soil, food and biota) by pesticides may lead to health problems for human and other non-target organisms in the environment [18]. Even though insecticide is one of the very important inputs in agriculture to prevent loss of production, but if not properly managed they could create major environmental and human health risks [26]. These risks could be high particularly for those occupationally exposed [27]. Occupational insecticides exposure can occur directly during mixing and DDT application and indirectly while performing re-entry tasks in pesticidetreated crops or by take home exposure. Insecticide’s exposure can occur through the skin (dermal uptake), via the respiratory system (inhalation), or via the mouth (ingestion) and may result in health effects like visual, dermal, cardiovascular, gastrointestinal, carcinogenic, endocrine disruption, developmental, neurological, and respiratory effects [28,29]. Majority of the farmers in Ethiopia do not properly wear protective measures and negligible use of protective clothing, among small-scale farmers in Ethiopia [30].
DDT Impact on Environment
DDT is highly persistent in the environment. The DDT half-life in soil is from 2 to 15 years (ATSDR, 1994). The half-life of DDT in an aquatic environment is about 150 years [31]. DDT is carried by rain into underground waters or other water supplies in the ecological system when applied to fields. The levels of DDT in water content might not be high, but aquatic organisms, especially zooplankton absorb them and reach very high levels. DDT level slowly raises within the food chain. The fishes that feed on zooplankton have higher concentrations and birds are the most damaged species by DDT. When mother is threatening the eggs of birds are born devoid of eggshell to protect them and the shell is too weak to support the weight and the eggs hatch before the appropriate time [32]. DDE is more persistent than DDT in the environment and has caused serious environmental problems worldwide.
DDT Impact on Human Health and Animal
At low environmental doses, the DDT effects on human heaths are unknown. After exposure to high doses, human symptoms can include vomiting, tremors or instability, and seizures. Laboratory animal studies showed effects on the liver and reproduction. DDT is considered a possible human carcinogen. Effects of DDT on human health and the environment depend on the dose of DDT and the timespan and frequency of exposure. It effects also depend on the health of a person and certain environmental factors (WHO, 1989). There is an enormous anxiety on human health hazards of DDT [33]. DDT is a persistent organic pollutant chemical in both biotic and abiotic environment and its residue can be detected in almost every human body [34]. The exposure of human beings to DDT can be in a utero, during breast feeding and through consumption of DDT with contaminated food [35]. The main route of human exposure to DDT is the dietary intake. The 90% of the DDT residue stored in a human body is because of consumption of different food items [36]. Many staple food items have been contaminated with DDT [37]. Indoor Residual Spraying (IRS) of pesticides for vector control may be another source of human exposure [38]. The dietary exposure may depend on the concentration of DDT intake and its metabolites in the food people eat and the amount of food consumed (ATSDR, 2002). Infants’ metabolic mechanism is not well matured to detoxify DDT. They are very susceptible when exposed to toxic pesticides compared to adults [39]. The infants’ food intake per body weight is higher [40]. In utero exposure to DDT has a potential risk on the infants’ neuro developmental growth at the age of 6-12 months [41]. Infants can also be exposed to DDT during breast milk feeding. A study done in India indicated that concentration of total DDT in breast milk for some infants were above the tolerable daily intake which will have a health risk [42]. Additionally, infants are also exposed to DDT when taking complementary homemade baby foods [43]. In Ethiopia, maize is the dominant cereal used in balancing food administered to infants after the age of 6 months [44]. However, maize can easily be affected by insects in the field and during storage [45]. To manage these insects, farmers use DDT which is banned for agricultural use. A recent study done in southwest Ethiopia indicated that, DDT was found above the Maximum Residue Limit (MRL) in maize samples collected from Jimma zone [37]. Infants consuming maize that contaminated with DDT and its metabolites may be face health risks. Pesticide application often results in residues in food which may cause a health risk for human [46]. Consumer risk assessment is an important step for regulation of pesticide use on food crops Hamilton et al. Regardless of all these problems, there is no study that has estimated the exposure and risks of infants to pesticides, particularly with DDT in Ethiopia. The current study contemplated to investigate the risk of total DDT for infants associated with consumption of maize in their complementary diets.
If female and male mice consumed doses of DDT for life, the males were twice as likely to develop liver tumors (WHO, 1979). DDT is slightly to moderately acutely toxic to mammals, including people. Through mammalian skin, DDT is poorly absorbed but, through an insect’s outer covering it is easily [3]. DDT exposed laboratory animals develop hyperexcitability, tremors, in coordination, and convulsions (WHO, 1979). The EPA has classified DDT as a B2 carcinogen [47]. It means DDT can cause cancer in laboratory animals, but there is insufficient or no evidence which shows that it may cause cancer in humans (WHO, 1979). Workers employed at a DDT manufacturing facility studied for 19 years did not develop cancer (WHO, 1979). In women exposed to DDT there is no correlation between increased risks of breast cancer [48]. Animals given potentially fatal doses of DDT develop liver lesions and those given DDT over a long period of time develop liver changes (WHO, 1979). DDT is converted into several breakdown products called metabolites in the body, such as Dichlorodiphenyldichloroethene (DDE). DDT and DDE are accumulated in the body’s fatty tissues. CDC scientists measured DDT and its metabolite DDE in the serum and estimate amounts of these chemicals that have entered people’s bodies. A small portion of the population had measurable DDT. Most of the population had detectable DDE. DDE stays in the body longer than DDT and DDE is an indicator of past exposure (CDC, 2009).
The reproductive or birth effects of DDT
Animal health effects from DDT, dogs fed DDT in low doses do not have reproductive effects (WHO, 1979). Rats become sterile after being fed DDT [49]. Mice fed low levels of DDT have embryos that fail to attach to the uterus and irregular reproductive cycles [49]. The offspring of mice fed DDT have a higher mortality rate (WHO, 1979). DDE causes thinning of eggshells in birds (EPA, 1975). DDE and DDT can pass to the fetus in pregnant women. Both chemicals are found in breast milk, resulting in exposure to nursing infants.
The accumulation of DDT in humans and animals
DDT tends to accumulate in the fatty tissues of insects, wildlife, and people, but produces no known toxic effects while it is stored in the fat [3]. DDT is metabolized into various breakdown products in the body including DDE, DDD and DDA When fat stores are used during periods of starvation the breakdown products of DDT are released into the blood where they may be toxic to the liver and the nervous system. Once DDT has accumulated in the body, it is excreted in the urine, feces, or breast milk. Breast milk is often used to measure a population’s exposure to DDT [3].
Biomagnification
DDT’s has chemical properties and the tendency to accumulate in animals. Lower animals on the food chain are eaten by other animals higher up; DDT becomes concentrated in the fatty tissues of predators (EPA, 1975). This continues until the primary predator of the food chain receives the highest dose, which may lead to adverse health effects. Once the use of DDT was discontinued in the U.S., its concentration in the environment and animals decreased.
The effects of DDT on wildlife
DDT is slightly to moderately toxic to birds when eaten (ATSDR, 1994). DDE decreases the reproductive rate of birds by causing eggshell thinning and embryo deaths (WHO, 1989). DDT is highly toxic to aquatic animals (WHO, 1989). DDT affects various systems in aquatic animals including the heart and brain (WHO, 1989). DDT is highly toxic to fish (WHO, 1989). Fish have a poor ability to detect DDT in water (WHO, 1989). DDT is moderately toxic to amphibians like frogs, toads, and salamanders. Immature amphibians are more sensitive to the effects of DDT than adults (WHO, 1989).
Role of Fungi in DDT Degradation
Biodegradation is carried out by microorganisms (bacteria and fungi) that naturally live in the environment. Fungi are often microscopic, but many can grow as long threads called hyphae or fruiting bodies like mushrooms, which can be visible to the naked eye. Fungi have very diverse metabolisms and use a wide variety of food and energy sources and perform many important functions in decomposition. Decomposers break down organic matter and recycle nutrients. The most decomposers are those that use aerobic respiration, using oxygen in the process of decomposition [5]. Microorganisms have a powerful enzymatic system which is responsible for the degradation of pollutants, but different microorganisms require a different substrate to metabolize pollutants. The results of the present research further confirmed the prevalence of co-metabolism in pollutant degradation by microorganisms [50]. DDT was classified as a compound with high pollutant and high environmental risk in the precedent-controlled product directory in Ethiopia. DDE is one of the toxic and primary metabolites of DDT and it is stable, easily bioaccumulated and difficult to biologically degrade in nature. The conversion of DDT to DDE by microorganisms has been termed a ‘dead-end side reaction and there have been few reports on the biodegradation of DDE. A study of the bacterial degradation system of DDE is useful for the development of effective bioremediation technologies for DDE. DDE was shown to cause serious environmental problems. DDT is persistent and degraded extremely slowly in the natural environment, the enhancement of its degradation by microorganisms has been popular in recent decades. Some bacteria and fungi were isolated from the environment, such as Clostridium sp. [47]. Pseudoxanthomonas jiangsuensis sp. Two white rot fungi, wood rot fungi and litter-decomposing basidiomycetes.
Fungal Mechanisms of DDT Degradation
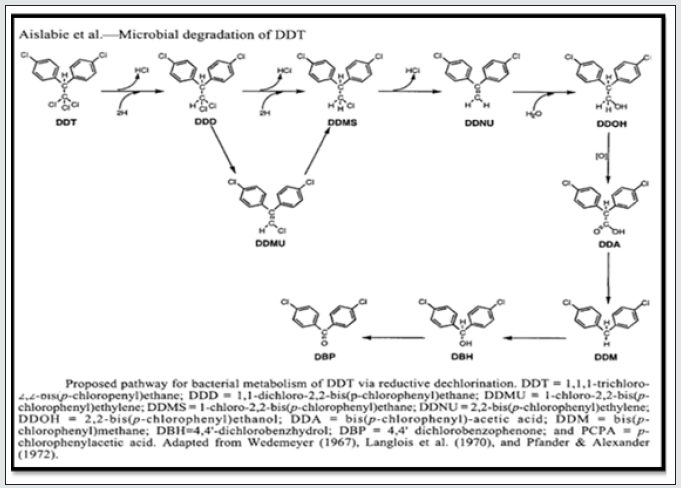
One mechanism for loss of DDT from soil is microbial degradation of DDT residues. The dominant reaction of dehydrochlorination of DDT to DDE is metabolic pathway of DDT by microorganisms, which is predominant under aerobic conditions and a reductive dechlorination to DDD under anaerobic conditions. The degradation of DDTr in soils is reliant on the presence and quantities of microbes in the contaminated soil with its degradative ability. These microbes may be inhabitant in the soil, or they may be isolated from elsewhere and introduced to the soil. Fungi are useful for the biodegradation of DDT that metabolize DDTr via reductive dechlorination, ligninolytic fungi which carry out ring cleavage of DDTr under aerobic conditions. Alternatively, microbes with the ability to degrade DDTr can be constructed using molecular techniques and introduced into the contaminated soil [51]. Transformation of DDT to DDD occurs readily in spiked soils under certain conditions. The process may be attributed directly to microbial activity, either bacterial or fungal [52], or indirectly to the generation of anaerobic conditions and/or the production and release of biomolecules that act as reductants, such as the iron porphyrins [53]. Since DDD accumulates in soil [54] subsequent biotransformation reactions, which lead to mineralization, occur very slowly. This could mean that microbes or microbial populations able to attack DDT directly and convert it to DBP via DDD are present in very low numbers Figure 2. Alternatively, environmental conditions may not allow biodegradation of DDD to proceed. It may therefore be necessary to introduce microbes to contaminated soils that are capable of mineralizing DDTr [55]. DDE could not further degrade but it could be metabolized into DDMU by reductive dechlorination with methanogens and biological sulfide available in the submarine [56]. Ligninolytic fungi for decontamination of soil have been described by [57]. These fungi have a broad spectrum of activity and are able to mineralize both DDT and DDE. The degradative enzymes are induced by environmental conditions rather than in response to the presence of the contaminant and being extracellular they degrade very insoluble chemicals. Almost all of the white-rot fungi secrete various extra cellular enzymes that degrade lignin, such as, Lignin Peroxidase (LiP), Manganese Peroxidase (MnP) and laccase. Presence of nonspecific enzyme system, enable fungi to degrade natural complex aromatic polymers of lignin as well as complex aromatic polymers that share structure with lignin, such as pesticides, PAHs, PCBs and dyes [58]. Although ligninolytic fungi can clearly degrade DDT their ability to do so in the environment is equivocal [59]. DDE could also degrade by the dechlorination enzymes, dioxygenase and hydrolase in the appropriate conditions. The dioxygenase and lignin peroxidase levels were higher with the addition of DDT, and these two enzymes play important roles in the degradation of DDT [60]. The further metabolism of DDE is mostly under aerobic conditions because there is a double bond structure of the relative instability in the molecule of DDE, and DDE is able to undergo oxidation [61]. The fungal strain Fusarium solani was able to metabolize DDT, DDD and DDE in the soil, but its products were not clear [62].
Current DDT Detoxification Approach
Bacterial degradation
To decontaminate the polluted sites the use of bacteria for the degradation and detoxification of numerous toxic chemicals such as insecticide is an effective tool. Isolation of indigenous bacteria capable of metabolizing DDT insecticide provides environmentally friendly means of in situ detoxification [63]. A diverse group of bacteria, including members of the genera Alcaligenes, Flavobacterium, Pseudomonas and Rhodococcus, metabolize DDT insecticide [64]. Actinomycetes have considerable potential for biotransformation and biodegradation of pesticides. Members of this group of Gram-positive bacteria have been found to degrade pesticides with widely different chemical structures, including organochlorine [64]. Co metabolism is addition of an easily metabolized organic matter such as glucose that increases biodegradation of recalcitrant compounds that are usually not used as carbon and energy sources degradations by microorganisms [65] and use of glucose as co-substrate increases the rate of biodegradation Swaminthan and Subrahmanyam, 2002.
Fungal degradation of DDT
Fungi can be screened out from soil and wastewater as an effective tool for biodegradation of toxic organic chemicals. Phanerochaete and related fungi that have the ability to attack wood possess a powerful extracellular enzyme that, acts on a broad array of organic compounds. The enzyme is a peroxidase (H2O2) produced by fungus, catalyzes a reaction that cleaves a surprising number of compounds. Culture of Phanerochaete chrysosporium, the most widely studied of these fungi for its biodegradative capacity, can degrade a number of Polycyclic Aromatic Hydrocarbons (PAHs) including pyrene, anthracene, di- and tribenzoic acids, several Polychlorinated Biphenyls (PCBs), 2,3,7,8-tetrachlorodibenzop- dioxin, DDT (Dichlorodiphenyltri-chloroethane), lindane, and chlordane Alexander, 1999. The transformations by fungus are slow, and a test of the biodegradation of DDT in soil pans failed to show an effect of Phanerochaete sordid in promoting bioremediation [66]. However, the addition of large inocula of this fungus resulted in an enhanced degradation of PCP as well as three- and four- but not five and six rings’ PAHs [67]. Another study performed by [68] showed a stimulation in the degradation of PCP by two species of Phanerochaete. Phanerochaete chrysosporium are utilized as a biodegradation of a wide variety of pollutants present in both liquid and soil cultures. P. chrysosporium is used as a model for studying the degradation of DDT Bumpus et al., 1985. Fungal technology appears promising for biodegradation of recalcitrant contaminants [68]. Fungi do not generally metabolize contaminants; degradation occurs extracellular by enzymes excreted by the fungi and systems, together with the translocation, bioaccumulation and contaminant storage/degradation. Plant-based soil remediation systems can be viewed as biological, solar-driven, pump and treat systems with an extensive, self-extending uptake network (the root system) that enhances the under-ground ecosystem for subsequent productive use [69-93].
Conclusion
DDT was so broadly used because of effective, relatively inexpensive to manufacture, and lasts a long time in the environment. It was disqualified because it persists in the environment, accumulates in fatty tissues, and can cause bad health effects on wildlife and human being. Biodegradation is carried out by microorganisms (bacteria and fungi) that naturally live in the environment. Bacteria and fungi have very diverse metabolisms: they use a wide variety of food and energy sources and perform many important functions. One especially important function is decomposition. Decomposers are bacteria and fungi that can break down organic matter and in doing so recycle nutrients. Each degradation step is catalyzed by specific enzyme produced by a degrading cell or enzyme found external to the cell. Degradation of insecticide by enzyme will stop at any step if an appropriate enzyme is not present. The insecticide had been intensively applied for agricultural pest control since 1940. The DDT half-life in soil is from 2 to 15 years. The half-life of DDT in an aquatic environment is about 150 years. DDT is carried by rain into underground waters or other water supplies in the ecological system when applied to fields. Effects of DDT on human health and the environment depend on the dose of DDT and the timespan and frequency of exposure. DDE and DDT can pass to the fetus in pregnant women. Both chemicals are found in breast milk, resulting in exposure to nursing infants. DDT tends to accumulate in the fatty tissues of insects, wildlife, and people, but produces no known toxic effects while it is stored in the fat. Microbes can be screened out from soil and wastewater as an effective tool for biodegradation of toxic organic chemicals. Phanerochaete and related fungi that have the ability to produce extracellular enzyme that, acts on DDT. Members of the genera Alcaligenes, Flavobacterium, Pseudomonas and Rhodococcus of bacteria metabolize DDT.
References
- Abiye A, Hadera G (2005) A report on POPs and other pesticides inventory in Ethiopia: POPs/N IP project. Addis Ababa: EPA/SEG.
- Agency for Toxic Substances and Disease Registry (1994) Toxicology Profile for 4,4’-DDT, 4,4’-DDE, 4,4’-DDD (Update). US Department of Human Health & Human Services.
- Agency for Toxic Substances and Disease Registry (2002) Toxicological Profile for DDT, DDE, Accessed 2015.
- ATSDR (2012) Agency for Toxic Substances and Disease Registry: Chemical and physical 448 information for DDT, DDE and DDD.
- Agrow (2006) World agchem market steady. AGROW 497 pp: 17.
- Akalu G, Taffesse S, Gunaratna NS, De Groote H (2010) The effectiveness of quality protein maize in improving the nutritional status of young children in the Ethiopian highlands. Food Nutr. Bull 31(3): 418-430.
- Akoto O, Andoh H, Darko G, Eshun K, Osei Fosu P (2013) Health risk assessment of pesticide residue in maize and cowpea from Ejura, Ghana. Chemosphere 92(1): 67-73.
- Alexander M (1994) Biodegradation and Bioremediation. Academic Press New York 274.
- Amare T, Abate A (2008) An assessment of the pesticide use, practice and hazards in Ethiopian rift valley. Institute for Sustainable Development. Ethiopia and Pesticide Action Network of the UK, Addis Ababa, Ethiopia.
- Aust SD (1994) Mechanisms white rot fungi use to degrade pollutants. Environmental science and technology 28: 78A-87A.
- Balkew M, Getachew A, Chibsa S, Olana D, Reithinger R, et al. (2012) Insecticide DP resistance: A challenge to malaria vector control in Ethiopia. Malar. J 11(1): 139.
- Beard J, Australian Rural Health Research Collaboration (2006) DDT and human health. Sci. Total Environ 355(1-3): 78-89.
- Bedi JS, Gill JPS, Aulakh RS, Kaur P, Sharma A, et al. (2013) Pesticide residues in human breast milk: Risk assessment for infants from Punjab, India. Sci. Total Environ 463-464, 720-726.
- Biscoe ML, Mutero CM, Kramer RA (2004) Current policy and status of DDT use for malaria control in Ethiopia, Uganda, Kenya and South Africa. International Water Management Institute, Colombo, Sri Lanka.
- Boul HL (1996) Effects of soil moisture on the fate of radio labeled DDT and DDE invitro. Chemosphere 32(5): 855-866.
- Brian B (2014) DDT Still Killing Birds in Michigan. A chemical plant-turned- Superfund site may be to blame. Environ Health News on July 28.
- Brodhagen M, Peyron M, Miles C, Inglis DA (2015) Biodegradable plastic agricultural.
- Bumpus JA, Aust SD (1987) Biodegradation of DDT [1,1,1-trichloro-2,2-bis (4-chloro-phenyl) ethane] by the white rot fungus Phanerochaete chrysosporium. Appl. environs microbiol 53(1): 2001-2008.
- Cameron MD, Timofeevski S, Aust SD (2000) Enzymology of Phanerochaete chrysosporium with Respect to the Degradation of Recalcitrant Compounds and Xenobiotics. Appl. Microbiol. Biotechnol 54(6): 751-758.
- Carson R (1962) Silent spring. Houghton Mifflin; Cambridge, Mass. Riverside Press. Boston. USA.
- Casals Casas C, Desvergne B (2011) Endocrine Disruptors: From Endocrine to Metabolic Disruption. Annu. Rev. Physiol 73: 135-162.
- Casida JE, Quistad GB (1998) Golden age of insecticide research: past, present, or future. Annu. Rev. Entomol 43: 1-16.
- Center for Disease Control and prevention (2009) Fourth national report on human exposure to environmental chemicals.
- Center for Food Safety (2006) Dietary exposure to DDT of secondary school students. The government of the Houng kong special administrative region, Food and Environmental Hygiene Department 43/F, Queensway Government Offices, 66 Queensway, Hong Kong.
- Chemicals Evaluated for Carcinogenic Potential (2006) Cancer Classification. Group E Evidence of Non-carcinogenicity for Humans-USEPA Office of Pesticide Programs, Health Effects Division, Science Information Management Branch.
- Cobbet CS (2000) Phytochelatins and their roles in heavy metal detoxification. J Plant Physiol 123(3): 825-832.
- Corona Cruz A, Gold Bouchot G, Gutierrez Rojas M (1999) Anaerobic-aero biodegradation of DDT (dichlorodiphenyl trichloroethane) in soils. Bull Environ Contam Toxicol 63(2): 219-225.
- Corvallis Oregon (1996) DDT Extension Toxicology Network (EXTOXNET). Oregon State University.
- Cousins IT, McLachlan MS, Jones KC (1998) Lack of an aging effect on the soil air partitioning of polychlorinated biphenyls. Environ. Sci. Technol 32(18): 2734-2740.
- Curtis CF (2002) Should the use of DDT be revived for malaria vector control? Biomedical (Bogota) 22(4): 455-461.
- Damalas CA, Eleftherohorinos IG (2011) Pesticide exposure, safety issues, and risk assessment indicators. Int J Environ Res Public Health 8(5): 1402-1419.
- Daston G, Faustman E, Ginsberg G, Fenner Crisp P, Olin S, et al. (2003) A Framework for Assessing Risks to Children from Exposure to Environmental Agents. Environ. Health Perspect 112(2): 238-256.
- Donaldson SG, Van Oostdam J, Tikhonov C, Armstrong B, Ayotte P, et al. (2010) Environmental contaminants and human health in Canadian Arctic. Sci. Total Environ 408(22): 5165-5234.
- Environment and Social Assessment International (2006) Pesticide action network UK. Pesticide use, Accumulations and Impacts: A case study in the rift valley.
- EPA (2004) Environmental Impact Assessment guideline on Pesticides. The Federal Environmental Protection Agency.
- Environmental Protection Agency, Office of Pesticide Programs, U.S. Government Printing Office. (1975) Washington, DC. DDT. A Review of Scientific and Economic Aspects of the Decision to Ban its Use as a Pesticide, EPA 540: 1-75-022. .
- Eskenazi B, Chevrier J, Rosas LG, Anderson HA, Bornman MS, et al. (2009) 'The Pine River Statement: Human health consequences of DDT use. Environ. Health Perspect 117(9): 1359-1367.
- Eskenazi B, Marks AR, Bradman A, Fenster L, Johnson C, et al. (2006) In Utero Exposure to Dichlorodiphenyltrichloroethane (DDT) and Dichlorodiphenyldichloroethylene (DDE) and Neurodevelopment among Young Mexican American Children. PEDIATRICS 118(1): 233-241.
- Ethiopian Biodiversity Institute (2015) Ethiopia's National Biodiversity Strategy and Action plan.
- Fernando T, Aust SD, Bumpus JA (1989) Effects of culture parameters on DDT [1,1,1 trichloro-2,2-bis (4-chlorophenyl) ethane] biodegradation by Phanerochaete chrysosporium. Chemosphere 19(8-9): 1387-1398.
- Glass BL (1972) Relation between the degradation of DDT and the iron redox system in soils. J. agricultural and food chemistry 20(2): 324-327.
- Glaser JA, Lamar RT (1995) Lignin degrading fungi as degraders of pentachlorophenol and creosote in soil. In: Bioremediation: Science and Applications. SSSA Special Pub 43: 117-133.
- Golovleva L, Pertsova R, Boronin A, Grischenkov V, Baskunov BP, et al. (1982) Degradation of polyaromatic insecticides by Pseudomonas aeruginosa containing bio- degradation plasmids. Microbiologia 51(6): 973-978.
- International Development Advisory Board (1956) Malaria Eradication. Report and Recommendations of the International Development Advisory Board. Inter-national Cooperation Agency, Washington, DC, USA.
- Hickey WJ (1999) Transformation and of fate of polychlorinated biphenyls in soil and sediment. In Adriano DC, Bollag JM, Frankenburger WT, Sims RC, Bioremediation contaminated soils. Agron. Monograph 37: 213-237.
- Jeong Y, Lee S, Kim S, Choi SD, Park J, et al. (2014) Occurrence and exposure assessment of polychlorinated biphenyls and organochlorine pesticides from homemade baby food in Korea. Sci. Total Environ 470-471: 1370-1375.
- Jusko TA, Klebanoff MA, Brock JW, Longneck MP (2012) In-Utero Exposure to Dichlorodiphenyltrichloroethane and Cognitive Development among Infants and School aged Children. Epidemiology 23(5): 689-698.
- Kearney PC, Woolson JR, Plimmer JR, Isensee AR (1969) Decontamination of pesticides in soil. Pesticides review 29: 137-149.
- Kidwell JM, LJ Phillips, Burchard GF (1995) Comparative analyses of contaminant levels in bottom feeding and predatory fish using the National Contaminant Biomonitoring Program data. Bulletin of Environmental Contamination and Toxicology 54(6): 919-923.
- Klaassen CD, Amdur MO, Doull J (1996) Eds. McGraw-Hill: New York. Casarett & Doull’s toxicology. The basic science of poisons, Fifth edition pp: 1056.
- Litsios S (1996) The Tomorrow of Malaria, Wellington, New Zealand. Pacific Press.
- McCauley LA, Anger WK, Keifer M (2006) Studying health outcomes in farm Workers population exposed to Pesticides. Environ Health Perspect 114(6): 953-960.
- Mekonen S, Ambelu A, Spanoghe P (2014) Zone: Pesticide residues in Pesticide residue evaluation in major staple food items of Ethiopia using the QuEChERS method: A case study from the Jimma food items. Environ. Toxicol. Chem 33(6): 1294-1302.
- Mervat SM (2009) Degradation of methomyl by the novel bacterial strain Stenotrophomonas maltophilia M1. Elect J Biotech 4: 1-6.
- Metcalf RL (1973) A century of DDT. J Agric Food Chem 21(4): 511-519.
- Ministry of Health (2006) National five-year strategic plan for malaria prevention and control in Ethiopia 2006-2010.
- Mischke T, brunette K, Acosta V, Weaver D, Brown M (1985) Agriculture source of DDT residues California’s environment. California, Department of Food and Agriculture, California, USA.
- Mitra J, Mukherjee PK, Kale SP, Murthy NBK (2001) Bioremediation of DDT in soil by genetically improved fungus Fusarium solani. Biodegradation 12: 235-245.
- Mulchandani A, Kaneva I, Chen W (1999) Detoxification of organophosphate pesticides by immobilized Escherichia coli expressing organophosphorus hydrolase on cell surface. Biotechnol. Bioeng 5: 436-440.
- Nadeau LJ, Menn FM, Breen A, Sayler GS (1994) Aerobic degradation of 1,1,1-trichloro-2, 2-bis (4-chlorophenyl) ethane (DDT) by Alcaligenes eutrophus A5. Applied and environmental microbiology 60(1): 51-55.
- Ngowi AVF (2003) A study of farmers’ knowledge, attitude, and experience in the use of pesticides in coffee farming. Afr Newsletter Occup Health Safety 13: 62-64.
- Ntzani EE, Chondrogiorgi M, Ntritsos G (2013) Literature review on epidemiological studies linking expo-sure to pesticides and health effects. European Food Safety Authority supporting publication 10(10): 497.
- Lamar RT, Davis MW, Dietrich DM, Glaser JA (1994) Treatment of a pentachlorophenol and creosote-contaminated soil using the lignin degrading fungus Phanerochaete sordida: A field demonstration. Soil Biol. Biochem 26(12): 1603-1611.
- Prescot LM, Harley JP, Klein (2002) Microbiology (5th Ed). The McGraw-Hill companies. Inc, North America.
- Pretty J, Hine R (2005) Pesticide Use and the Renvironment. Earthscan, London, UK.
- Quensen JF, Tiedje JM, Jain MK (2001) Factors controlling the rate of DDE dechlorination to DDMU in Palos Verdes margin sediments under anaerobic conditions. Environ Sci Technol 35(2): 286-291.
- Roberts DR, Andre RG (1994) Insecticide resistance issues in vector borne disease control. Am J Trop Med Hyg 6: 21-34.
- Sadasivaiah S, Tozan Y, Breman JG (2007) Dichlorodiphenyltrichloroethane (DDT) for indoor residual spraying in Africa: how can it be used for malaria control? Am. J. Trop. Med. Hyg 77(6 supply): 249-263.
- Safe SH (1998) Interactions between hormones and chemicals in breast cancer. Annu. Rev. Pharmacol. Toxicol 38: 121-158.
- Safferman SI, Lamar RT, Vonderhaar SS, Neogy R, Haught RC, et al. (1995) Treatability study using Phanerochaete sordida for the bioremediation of DDT contaminated soil. Toxicol Environ Chem 50: 237-251.
- Sari AA, Tachibana S, Muryanto A (2012) Correlation of ligninolytic enzymes from the newly found species Trametes versicolor U97 with RBBR depolarization and DDT degradation. Water Air Soil Pollution 223: 5781-5792.
- Singh DK (2008) Biodegradation and bioremediation of pesticide in soil: concept, method and recent developments. Indian J. Microbiol 48(1): 35-40.
- Sori W, Ayana A (2012) Storage pests of maize and their status in Jimma Zone, Ethiopia. Afr J Agric Res 7(28): 4056-4066.
- Stockholm Convention on Persistent Organic Pollutants (2001) Convention Text. Geneva: United Nations Environment Programme.
- SubbaRao RV, Alexander M (1985) Bacterial and fungal co metabolism of 1,1,1-trichloro-2,2-bis (p- chlorophenyl) ethane (DDT) and its breakdown products. Applied and environmental micro-biology 49(3): 509-516.
- Suspended, canceled and restricted use pesticides EPA (1990) 20T-1002. U.S. Environmental Protection Agency, Office of Pesticide Programs, U.S. Government Printing Office. Washington, DC, USA.
- Taverne J (1999) DDT to ban or not to ban? Parasitol Today 15(5): 180-181.
- The US EPA (1997) Reference Dose Tracking Report US. Environmental Protection Agency. Washington, DC, USA.
- Toxicology Profile for 4,4’-DDT, 4,4’-DDE, 4,4’-DDD (Update). (1994) U.S. Department of Human Health &Human Services, Agency for Toxic Substances and Disease Registry.
- USEPA-United States of Environmental Protection Agency (1997) Special Report on Environmental Endocrine Disruption: An Effects Assessment and Analysis, U.S. Environ‐mental Protection Agency, Report No. EPA/630/R-96/012, Washington DC, USA.
- Vall O, Gomez Culebras M, Puig C, Rodriguez Carrasco E, Gomez Baltazar A, et al. (2014) Prenatal and Postnatal Exposure to DDT by Breast Milk Analysis in Canary Islands 9(1): e83831.
- Van Ert M, Sullivan JB (1992) Organochlorine Pesticides in Sullivan JB and Krieger GR, Hazardous Materials Toxicology, Clinical Principles of Environmental Health. Williams & Wilkins, Baltimore, MD.
- Vergucht, De Voghel, Misson (2006) Health and environmental effects of pesticides and type 18 biocides (HEEPEBI).
- Vishan I, Sivaprakasam S, Kalamdhad A (2017) Isolation and identification of bacteria from Rotary drum compost of water hyacinth. Int. J. Recycl Org Waste Agric 6: 245-253.
- Walter M (1992) Biodegradation of DDT and DDE. Unpublished MSc thesis, Lincoln University, New Zealand.
- Westbom R, Hussen A, Megersa N, Retta N, Mathiasson L, et al. (2008) Assessment of organochlorine pesticide pollution in Upper Awash Ethiopian state farm soils using selective pressurized liquid extraction. Chemosphere 72(8): 1181-1187.
- Williamson S, Ball A, Pretty J (2008) Trends in pesticide use and drivers for safer pest management in four African countries. Crop Protection 27(10): 1327-1334.
- Wolff MS, Toniolo PG, Lee EW, Rivera M, Dubin N (1993) Blood levels of organo-chlorine residues and risk of breast cancer. J Natl Cancer Inst 8: 648-652.
- World Health Organization (1989) DDT and its derivatives. Environmental aspects. Environmental Health Criteria. Geneva, Switzerland p. 83.
- World Health Organization (1979) DDT and its derivatives. Environmental Health Criteria. Geneva, Switzerland p. 9.
- Xu B, Jianying G, Yongxi Z, Haibo L (1994) Behaviour of DDT in Chinese tropical soils. J. environmental science and health part B 29: 37-46.
- Yewhalaw D, Wassie F, Steurbaut W, Spanoghe P, Van Bortel W, et al. (2011) Multiple Insecticide Resistance: An Impediment to Insecticide-Based Malaria Vector Control Program 6(1): e16066.
- Zoro JA, Hunter JM, Eglinton G, Ware GC (1974) Degradation of p, p’-DDT in reducing environments. Nature 247: 235-237.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...