Lupine Publishers Group
Lupine Publishers
Menu
Mini Review(ISSN: 2641-6875) 
Feline Leishmaniasis Diagnostic Algorithm Volume 3 - Issue 4
Sandra López Fernández1*, Carmen Chicharro2, Javier Nieto2, Fernando Fariñas Guerrero3, Eduardo Martínez Manzanares1 , Encarnación Clavijo Frutos1 and Eugenia Carrillo2
- 1Department of Microbiology, Faculty of Medicine, University of Malaga, Spain
- 2Leishmaniasis and Chagas Disease Unit, Centro Nacional de Microbiología, Instituto de Salud Carlos III, Spain
- 3Institute of Clinical Immunology and Infectious Diseases, Grupo YNMUN Biomedicine, 29018 Malaga, Spain
Received:May 29, 2023; Published: June 21, 2023
*Corresponding author: Sandra López Fernández, Department of Microbiology, Faculty of Medicine, University of Malaga, Spain
DOI: 10.32474/CTBM.2023.03.000170
Abstract
Feline Leishmaniasis has been underestimated for a long time, but recent studies have shown that cats are susceptible to getting infected and develop the infection or keep blood-borne pathogen for indeterminate time. Due to this, veterinary practitioner has not reliable methods available to identifying and diagnosing Leishmaniasis in cats. In this research, more than five hundred cats were collecting samples from blood, tissues and health checks and all results were studied to develop a diagnostic algorithm to facilitate their work and discover new cases.
Keywords: Feline leishmaniasis; leishmania infantum; cats sick with leishmaniasis; leishmaniasis in cats
Background
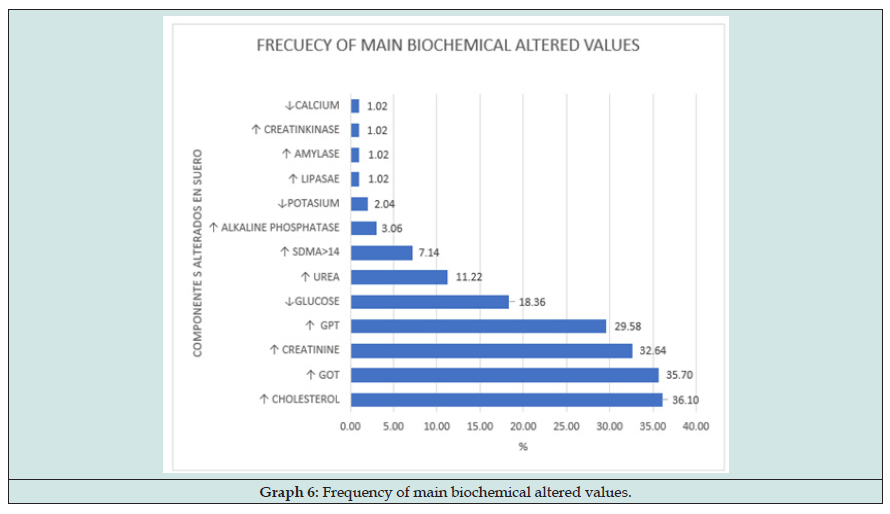
One of the main challenges for Veterinary practitioner in assessing active infection by Leishmania infantum [1] in cats is to determine what alterations he must find in the tests performed. Although some parameter of this infection can be extrapolated to other species such as dogs or Humans [2,3], because natural resistance of cats to the clinical development and it is provided on numerous occasions by coinfections and/or underlying diseases, the study of examination testing can be complicated. Taking as an example the blood level of eosinophils, it is common in parasite diseases, and it are described both in humans and in dogs, but eosinophilia cannot be orientated if the cat is infected by other parasites or pathogens. Another parameter that exemplifies the difficulties entailed in Feline leishmaniasis is the interpretation of neutrophils levels in blood. Main cells dedicated to phagocytosis (polymorph-nucleated or granulocytes) are Neutrophils, who act like cellular defence. It is formed in bone marrow where some of them stay being reserved for future infections showing their nucleus in crook form while others mature forms with segmented nucleus go to blood and tissues to fight against pathogens. High level of neutrophils can be success in hepatic injury, and liver is target-organ for L. infantum, so high neutrophils and high transaminases in blood are reason enough to included Leishmaniasis in the differential diagnosis. But on the other hand, low level of neutrophils in blood can be caused by retroviral infections in cats like FIV or FeL, for what neutropenia can hide Leishmaniasis infection if the cat is sick with FeL or FIV. Due to these difficulties, it becomes necessary look for a common pattern in cat infected by L. infantum and discover which abnormalities can be found in clinical or not clinical infections and compare with results obtained by other diseases sings. This can be complicated studying out-door cats because they are exposed to more pathogens that pet cats who live in-door.
Materials and Methods
Assuming that, despite the differences of cats compared with dogs or Humans, some clinical and analytic disorders derived from physiopathology of Leishmaniasis infection, that are similar [4- 7] than other species (Th1 or Th2 defense), the cat could follow a pattern like dog model, a study has been designed to include all altered variables in Humans or Dogs [8-12]. The statistical treatment consists in comparing the expected with the observed frequencies of each distribution, according to the hypothesis made before the beginning of the study. Over seven years Samples of 528 cats have been collected from Costa del Sol and Costa Tropical in South of Spain. All cats were sedated and anesthetized properly. under aseptic conditions, blood sample were collected from jugular vein and after putting into EDTA, Li-Heparin and dry tubes for Hemogram, Biochemical, ionogram, Proteinogram and Serology (IFAT, Lateral flow). One drop of fresh blood was deposited on a slide glass for Microscopy.
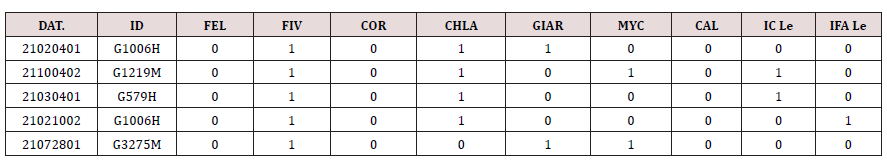
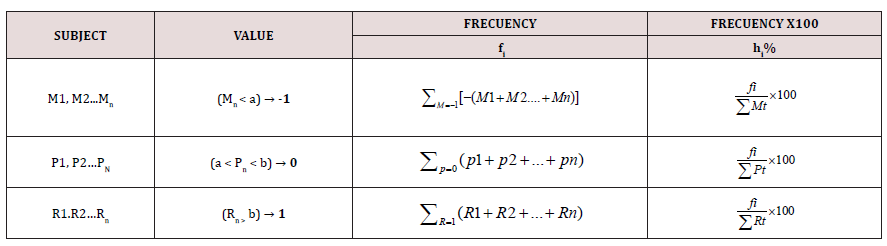
Results were performance with 3 types of value, -1 for negative results (e.g., lymphopenia), 0 for physiological or normal results (e.g., Potassium normal) and 1 for higher physiological level (e.g., hypergammaglobulinemia), in this way, frequency values were particularly useful to calculate. However, other pathogens detected have only 2 values, 0 (no detected) or 1 (antigen or antibody detected) (Table 1). Samples from other tissues were collected for Molecular detection and Microscopy [13,14] observations. Bone marrow, lymph nodes and spleen samples were collected with FNPA (Fine-needle puncture-aspiration technique). Bone Marrow from articulation, Lymph nodes from popliteus or cervical nodes, spleen with ultrasound-guided aspiration in only 100 subjects (owners or responses did not authorized the technique in other cats. Samples from mucous membrane and skin were collected using Deep technique, mucous membrane from nose and/or lips angle and skin from peek of ears (common place of sandfly bites). Half of the subjects were tested with molecular technique (PCR) in all their tissues. PCR [15,16] tests were done from DNA obtained digesting the tissues with commercial process Biotools® and amplified with the protocol LnPCR (nested PCR) which amplified all Leishmania Spp. Target: Mitochondrial gen- SSUrRNA.
PCR MIX conditions:
Table 1. Other pathogens table. Dat: collection day and number, ID, identification, FEL Feline Leukemia, COR Feline Coronavirus, CHLA Chlamydia, GIAR giardia Spp. MYC mycoplasma, CAL Feline calicivirus, IC Le Feline Leishmaniasis lateral Flow, IFA Le Feline leishmaniasis Indirect Immunofluorescence assay.
R1: 5´-GGT TCC TTT CCT GAT TTA CG-3´ R3: 5´-TCC CAT CGC AAC CTC GGT T- 3´
R2: 5´-GGC CGG TAA AGG CCG AAT AG-3´ R4: 5´-AAA GCG GGC GCG GTG CTG–3´
Each sample was fixed over slide glass and stained with Diff- Quick®. The tissues were kept in NET19 crioprotector buffer, one copy in Málaga university and other copy in ISCIII. Previously, all subjects were examined detecting all health parameters and wrote in the same number code -1, 0, 1. Finally, frequency values were added in a contingence square pair by pair to calculate the relationship (chi2) to design the algorithm for diagnoses (Table 2).
Table 2. N population, M, P, R variables value every single subject for each concept. “a” and “b” max. and min. delimit ranges of values (healthy values). Over “b” value, code is -1, between (ab) ̅ value is = and under “a” value is -1. fi is the frequency and hi is %frequency.


Null Hypothesis, no dependent Vs Alternative Hypothesis, dependent
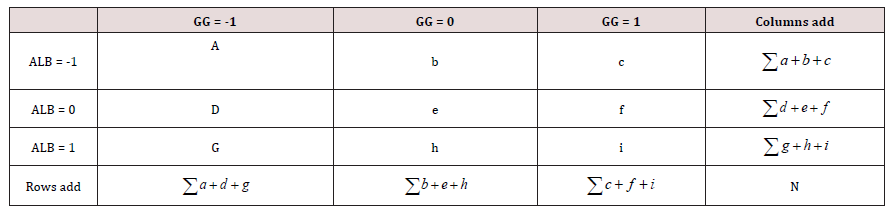
Example in a contingence table: albumin (ALB) Vs Gamma globulin (GG): (Table 3)

Check X2c critical chi-square, knowing data are r =3 rows and α=3 columns, we calculated the degree of freedom k.
k=(r-1)×(α-1) = 4
level of significance: 5%, Confidence: 95, so α=0.05 and P1=0.95 And faced:

Results
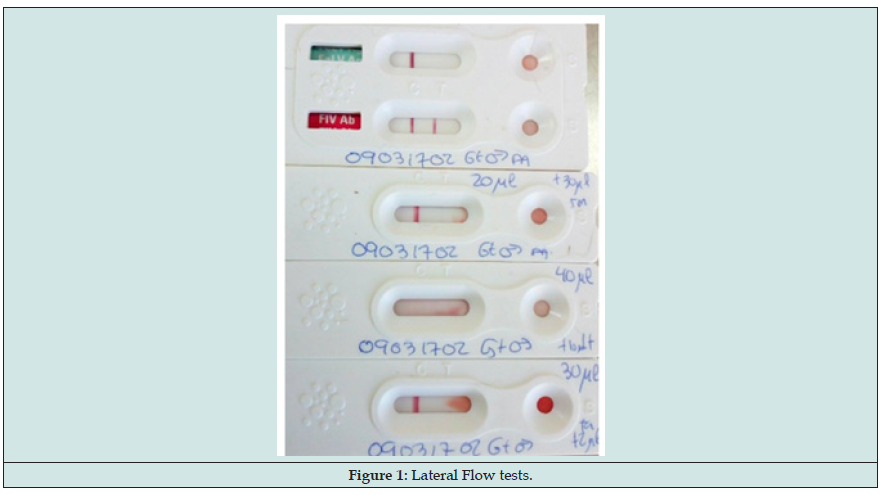
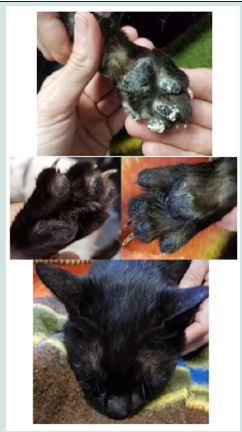
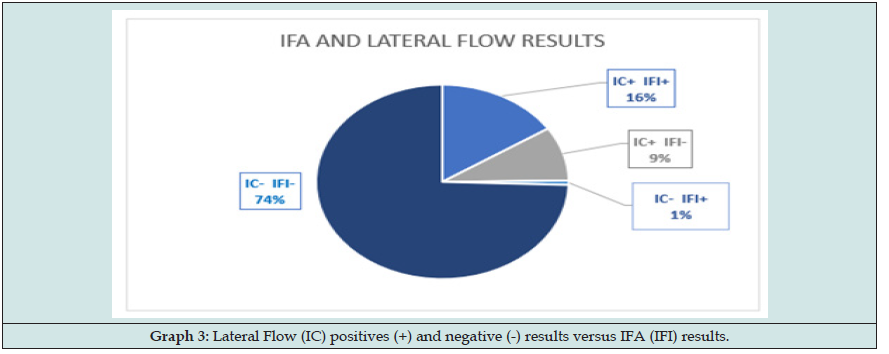
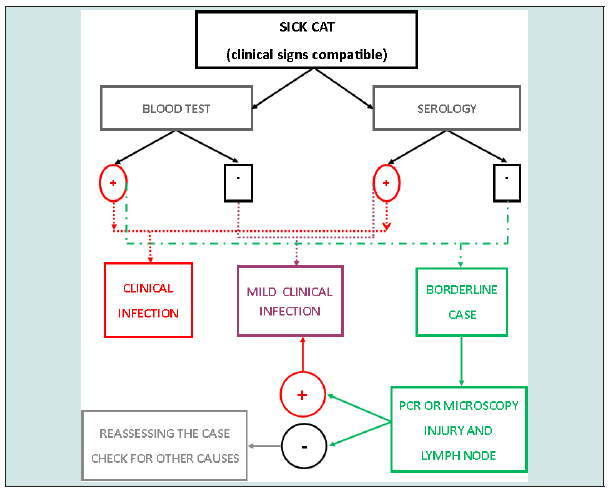
To design and develop the diagnostic algorithm we have started from Canine and Human main sings and look for similar disturb in cats. This is what we found (Graphs 1-6) (Figures 1-3): Here we found that usual dilution limit (1/80) used to diagnose dogs infected does not work with cats, our Lateral Flow technique (developing in this moment by Commercial laboratory URANOLAB ®) were positive in 1/20 and 1/40 IFA dilutions, exactly the subjects with skin nodules PCR or microscopy positives to L. infantum.Main diagnosis technique was by skin nodules identification, and other skin injuries like chancres, flaking and breaks. Samples collecting techniques were deep skin scrapings and fine needle aspiration cytology (Figure 3).
Figure 4: Distribution of clinical sings frequency found in infected subjects (Par. Identification or Serological results).
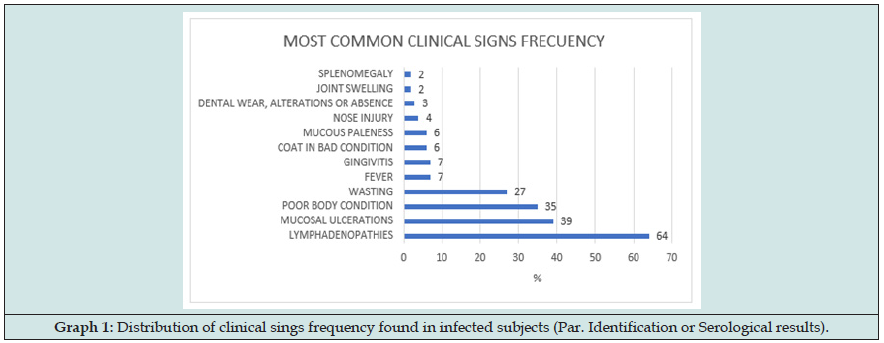
Figure 7: Hemogram abnormalities. The observed alterations were anisocytosis, toxic shift in neutrophils, Dohle bodies, leukocytes degeneration, acanthosis, schistocytes, cytoplasmic vacuolization and Rouleaux formations.
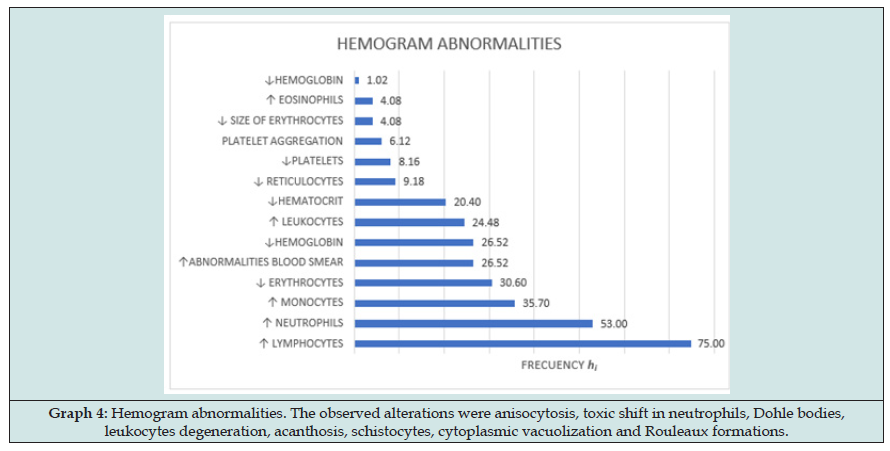
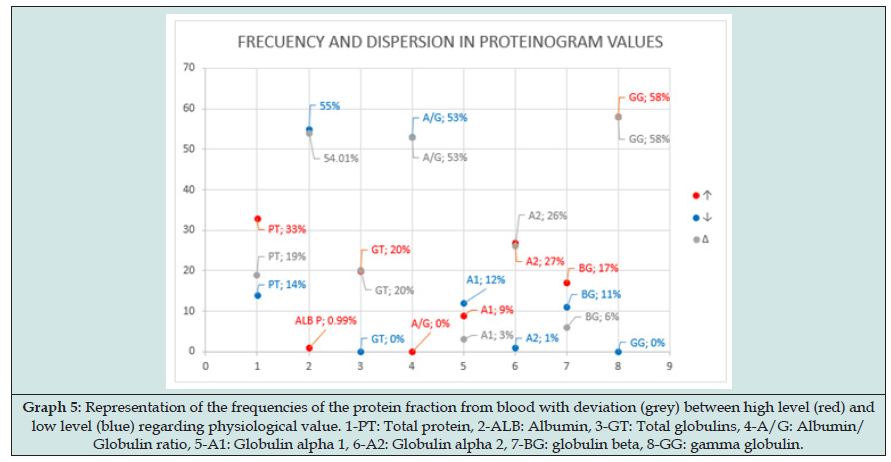
Figure 8: Representation of the frequencies of the protein fraction from blood with deviation (grey) between high level (red) and low level (blue) regarding physiological value. 1-PT: Total protein, 2-ALB: Albumin, 3-GT: Total globulins, 4-A/G: Albumin/ Globulin ratio, 5-A1: Globulin alpha 1, 6-A2: Globulin alpha 2, 7-BG: globulin beta, 8-GG: gamma globulin.
Discussion
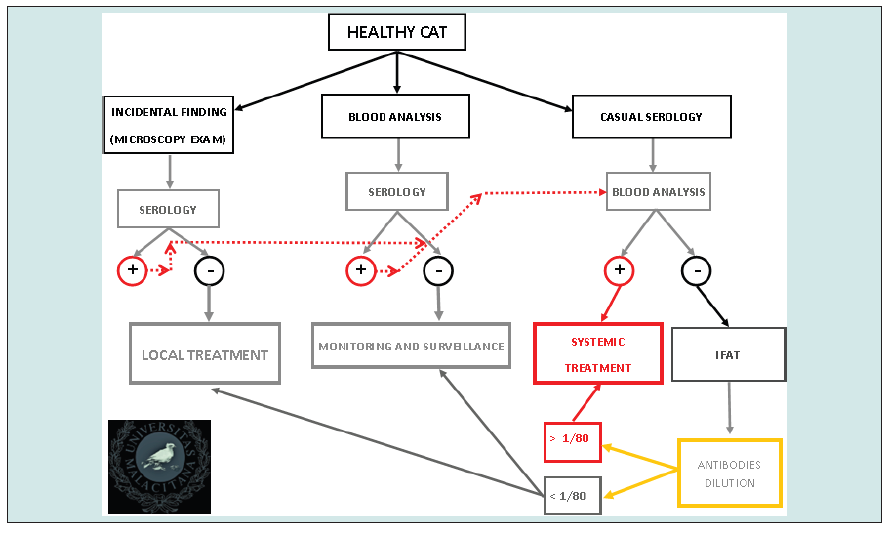
The diagnostic approach in cats can begging in two ways. Firstly, subjects without clinical sings so they are not Leishmaniasis infection suspect status. They are cats who are in contact with the parasite and are beginning the defense status (effective defense or sick) or skin primary status that does not show other sings. In the other hand, there are cats with clinical sings that can lead to suspicion Leishmaniasis.
Cats Apparent Healthy
Clinical sings are not evident or are so mild that the owner has not given importance to them. In this group of subjects, we can see light cutaneous reactions, chancres, uveitis, and the patient must be controlled conducting deep scraping of the skin and injuries and studied with PCR or microscopy techniques. When uveitis is present without other sings, the differential diagnosis must include Leishmaniasis. In these cases, we can find positive results in PCR or microscopy but not serology or analytic alterations, this therefore does not discount clinical infection. It could mean that the cats in the early infection. It is impossible to determine the future evolution in this stage, so the animal must be monitored for the next weeks and months. On way to make this control is punction of lymph node and blood test 2 months after to check if the infections has been controlled or disease progressed. This patient must have local treatment in the lesioned points and one immunomodulatory treatment that help its immune system to fight parasites that could arrive to blood or lymphatic circuit. Cats with uveitis but serological negative results must be monitored two months after. Other type of finding can be skin injuries no related by the veterinarian practitioner with Leishmania spp. But in a laboratorial skin test (deep scratch, biopsy) Amastigotes can be observed. Other points must be checked, and complete analysis is the next step to take. We must be careful with negative results so L. infantum can appears or not in other places and negative results does not exclude infection.
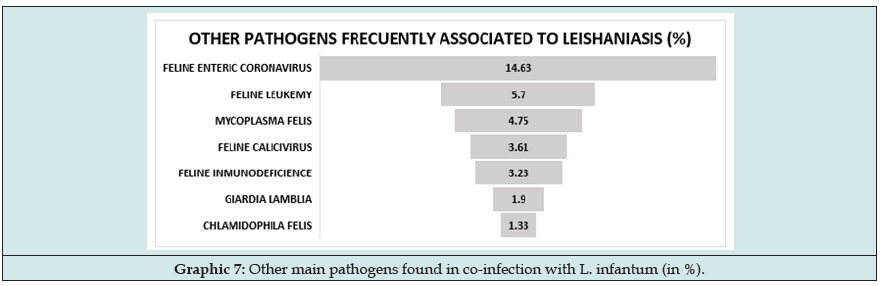
Eventually, we can find patient who are healthy subjected to periodic check-ups and the results show compatible with the disease abnormalities. Next step is serology and look for clinical sings of infection. European Laboratory are working to develop fast lateral flow test with semi quantitative serology so cats who live in endemic areas should be checked every year. For patients in whom are laboratorial + clinical + serology positive does not need PCR or other parasite diagnosis this justified systemic treatment. It should be noted that the presence of other pathogens can impair immune response so one infected but nod sick cat develops the active infection or, in the opposite case, cat sick with another disorder is bitten and infected showing inadequate immune response. Most common pathogens associated to Leishmaniasis has been retrovirus (FeL, FIV) but in this researching we found more frequency in the association L. infantum and Feline Enteric Coronavirus (Graph 7).
Sick cats
Subjects with weight loss, skin injuries, lymphadenitis, splenomegaly, gingivitis, mucosal pale, nose injuries, conjunctivitis, fever, non-regenerative or low-regenerative anemia, should be considered “suspect cases” and research analytic disturbs also parasite and microscopy identification. It should be noted that Quantitative serology IFAT technique can show dilutions data 1/20 to 1/80 that can be initial infection or skin form. If the parasite is found, local treatment must be done and cat be monitored in the future, as well IFAT low dilutions results.
Conclusion
Funding
This study was supported by Health Institute Carlos III (Madrid), Uranolab® Laboratory (Barcelona) and Málaga University (Malaga).
Interest Conflict
Not pertinent
Animal Experimentation
No pertinent, all samples were collected in a veterinary clinical procedure, habitual and respecting animal welfare.
References
- Fariñas F, Sandra L (2018) Leishmaniosis: una visión general de la enfermedad. Clinifectovet Multimédica 1: 1-14.
- Velez R, Ballart C, Domenech E, Abras A, Fernández-Arévalo A, et al. (2019) Seroprevalence of canine L. infantum infection in the Mediterranean region and identification of risk factors. Prev Vet Med 162: 67-75.
- Guias OMS (2020) Leishmaniosis, www.WHO.ink/es/news-room/fact-sheet/detail/leishmaniasis.
- Manolis K Chatzis, Panagiotis G Xenoulis, Leonidas Leon tides, Dimitrios Kasabalis, Mathios E Mylonakis, et al. (2020) Evaluation of clinicopathological abnormalities in sick cats naturally infected by L. infantum. Helion Pub 6(10): e05177.
- Morsey TA, Michael SA, Disi AM (1980) Cats as reservorir hosts of human parasites in Amman, Jordan. Egypt. Soc Parasitol 10(1): 5-18.
- Navarro JA, J Sánchez, C Peñafiel-Verdú, A J Buendía, J Altimira, et al. (2010) Histopathological lesions in 15 Cats with Leishmaniosis. J Comp Path 143(4): 297-302.
- Manolis K Chatzis, Leonidas Leontides, Labrini V Athanasiou, Elias Papadopoulos, Dimitrios Kasabalis, et al. (2014) Evaluation of Indirect Inmunofluosforescence antibody test an Enzyme-linked Inmunosorbent Assay for the Diagnosis of Infection by L. infantum in Clinically normal and sick cats. Exp Parasitol 147: 54-59.
- Susan Little, Julie Levy, Katrin Hartmann, Regina Hofmann-Lehmann, Margaret Hosie, et al. (2020) AAFP Feline Retrovirus Testing and Management Guidelines. J Feline Med Surg 22(1): 5-30.
- Laia Solano-Gallego, Guadalupe Miró, Alek Koutinas, Luis Cardoso, Maria Grazia Pennisi, et al. (2011) LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors 4: 86.
- Maria G Pennisi (2015) Leishmaniosis of Companion Animals in Europe: An Update. Vet Parasitol 208(1-2): 35-47.
- Eva V, Annamaria P (2012) Stray Dog and cat Laws and Enforcemen in Czech Republic and Italy. An Ist Sanit 48(1): 97-104.
- Rougeron V, Catzeflis F, Hide M, De Meeûs T, Bañuls AL, et al. (2011) First Clinical Case of Cutaneous Leishmaniasis Due to L. braziliensis in a Domestic Cat from French Guaiana. Veterinary Parasitology 181(2-4): 325-328.
- Silvio F Guimarães Carvalho, Elenice Moreira Lemos, Ralph Corey, Reynaldo Dietze (2003) Performance of recombinant K39 antigen in the diagnosis of Brazilian visceral leishmaniasis. Am J Trop Med Hyg 68: 321-324.
- S Cortes, N Rolão, J Ramada, L Campino (2004) PCR as a rapid and sensitive tool in the diagnosis of human and canine leishmaniasis using Leishmania donovani s.l.-specific kinetoplastid primers. Trans R Soc Trop Med Hyg 98(1): 12-17.
- R Fisa, C Riera, M Gállego, J Manubens, M Portús, et al. (2001) Nested PCR for diagnosis of canine leishmaniasis in peripheral blood, lymph node and bone marrow aspirates. Vet Parasitol 99(2): 105-111.
- Chatzis MK, Andreadou M, Leontides L, Kasabalis D, Mylonakis M, et al. (2014) Cytological and molecular detection of Leishmania infantum in different tissues of clinically normal and sick cats. Vet Parasitol. 202(3-4): 217-225.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...