
Lupine Publishers Group
Lupine Publishers
Menu
Research Article(ISSN: 2641-6875) 
Does Seed Inoculation with PGPRs Affect Germination and Final Biomass of Flax Under Drought Stress Conditions? Volume 2 - Issue 4
Sanaz Rajabi Khamseh* and Abdolrazagh Danesh Shahraki
- Department of Agronomy, Faculty of Agriculture, Shahrekord University, Iran
Received:September 27, 2021; Published:November 16, 2021
*Corresponding author: Sanaz Rajabi Khamseh, Shahrekord University, Iran
DOI: 10.32474/CTBM.2021.02.000141
Abstract
Seed germination as a primary aspect of growth is sensitive to water deficit. The current experiments were carried out to test the effects of drought stress and plant growth promoting rhizobacteria (PGPR) inoculation on seed germination, seedling growth, and biomass production of flax. Initially, the efficacy of PGPR (control, Bacillus amyloliquefaciens, Bacillus sp. strain1, Bacillus sp. Strain2, Azotobacter chroococcum, Pseudomonas putida, and Azospirillum lipoferum) and drought stress (0, -0.75, -1.5, -2 and -2.5 bar of PEG-6000) were estimated on flax germination under laboratory conditions. Then, bacterial treatments for the pot experiment were selected based on the laboratory experiment results (individually and in combination). Drought stress levels in the pot experiment were included 50%, 75%, and 100% crop water requirement. Results showed that the seeds inoculated with PGPRs under drought conditions positively affected seed germination and seedling growth under laboratory conditions. On the other hand, in the pot, emergence properties, dry biomass production, and root-related traits of bacterial inoculated plants were also improved compared with controls. B. amyloliquefaciens, Bacillus sp. strain1, and A. chroococcum in laboratory and coapplication of mentioned rhizobacteria in the pot recorded pronounced impact on most of the traits. Moreover, bacterial inoculation is proved to be an effective technique to increase the performance, growth and final biomass production of plants under unfavorable conditions like drought stress.
Keywords: Azotobacter; Bacillus; Drought; Germination; Oilseed
Introduction
The seeds and oil of oilseeds, a rich source of bioactive compounds, have positive effects on disease prevention [1]. Flaxseed (Linum usitatissimum L.) as an oilseed crop has been used for formulation of healthy functional food [2] and non-food applications from ancient times to the present [3]. The nutritional importance of flax is for its proteins, lipids, and minerals. Its oil is also a significant source of omega-3 fatty acids, lignans, fiber, mucilage gums, and lignin [1]. The early establishment of seedlings is the most important stage in plants life cycle. Success germination guarantees plants survival and production. Biotic and abiotic stresses as limiting factors have destructive effects on growth and developmental process. Drought stress is one of the serious threats which influences germination, growth, and developmental process through non-normal physiological mechanisms [4]. Under laboratory conditions, polyethylene glycol (PEG) usually applies to induce drought stress, which is not likely to infiltrate into plant tissue quickly [5]. Reduced germination and establishment under water deficit conditions have been investigated in plethora of plants including wheat [6], maize [7], coffee [8], sorghum [9], and soybean [10]. Seeds priming as an alternate, unexpansive and practical method through metabolic process regulation can upgrade seed germination [11] under unfavorable conditions like drought stress. Seeds biopriming with microorganisms named Plant Growth Promoting Rhizobacteria (PGPR) via entering or adhering the seeds causes increase in germination rate and uniformity, high crop establishment, quality and quantity improvement [12]. Generally, mechanisms of these microorganisms for growth promotions are included growth substances secretion, antifungal compounds production, and induction of plant systematic resistance [13]. The capacity of Azotobacter and Bacillus strains to phytohormones production has been confirmed [14]. It has been reported that combined inoculation of bacteria improved wheat seedlings germination and vigor index of stress conditions [15]. [16] found that the germination rate of soybean plants improved by 50% under unfavorable environments. It was hypothesized that PGPR application can decrease destructive effects of drought stress in plants. Based on these considerations, the current research was planned and carry out to examine the effects of individual and combined applications of PGPRs on the establishment and production of flax under drought stress conditions.
Materials and Methods
Investigation of Bacterial Growth Promoting Properties
An agar medium comprising calcium phosphate was used as inorganic phosphate to determine the bacteria’s ability to solubilize phosphate. Bacteria were tested on a plate using the National Research Institute’s Phosphate (NBRIP) growth medium. PGPRs were cultured in NBRIP broth for four d and inoculated on NBRIP agar plates. A loop filled with each culture was then placed on the plates at 30°C for seven d. The appearance of halo zones around the colonies after five d indicated phosphate solubilization. The emerged halo zones helped to classify low (diameter = ˂ 1 cm), medium (diameter = 1-2 cm) and high phosphate solubilizer (diameter = ˂ 2 cm). In order to distinguish ammonia production capability, the strains were cultured in peptone water broth at 27°C for five days before 1 ml of the Nessler’s reagent was mixed in 0.2 ml of the culture supernatant, and then, its volume was made to 8.5 ml using ammonia-free distilled water. The alteration of solution color from brown to yellow was considered as ammonia production [17]. For Indole acetic acid production [18], bacterial strains were added to 100 ml of the nutrient broth under continuous shaking for two days at 25°C. Accordingly, the cultures moved to 50 ml falcon tubes and centrifuged at 3000g for 10 minutes before 2 ml of the Salkowski’s reagent was mixed with 1 ml of the supernatant. The Salkowski reagent was made through dissolving 4.5 g of FeCl3 in 1 L of concentrated (10.8 M) H2SO4. Reagent was added to the sterile nutrient broth as the control treatment. Indole acetic acid production was specified through solution color variation from yellow to brown. Chrome Azurol S (CAS) agar medium was used for siderophore production [19]. CAS agar plates were prepared by adding 100 ml CAS reagent to 900 ml Luria Bertani (LB) agar medium. Bacterial strains were then spot-inoculated separately on plates and maintain for six days in an incubator at 28°C. Noninoculated plate was used as the control treatment. An orange zone round the bacterial showed siderophore production [20].
Bacterial Preparation
Bacterial strains were gained from the Faculty of Agriculture, Shahrekord University. Single colonies of the bacteria were separately cultured in 250 ml Erlenmeyer containing 50 ml Trypticase Soy Broth and incubated at 32 ±4°C under continuous shaking for 48 hours before washing the bacterial cells three times with NaCl 0.85% in order to remove Trypticase Soy Broth residues. The suspensions were then diluted in NaCl 0.85% to 5×106 CFU. ml- 1Ultimately, surface sterilized seeds were inoculated using 150 ml of each bacterial culture for two hours before planting.
Experimental Factors and Investigated Traits
Laboratory tests
Using a factorial in RCBD design with three replications, laboratory tests were carried out at the Seed Technology Laboratory of Shahrekord University, Shahrekord, Iran. The factors involved bacterial strains (non-bacterial inoculation or control (C), Bacillus amyloliquefaciens (B1), Bacillus sp. strain1 (B2), Bacillus sp. strain2 (B3), Azotobacter chroococcum (A1), Pseudomonas putida (P), and Azospirillum lipoferum (A2)) and drought stress (0, -0.75, -1.5, -2 and -2.5 bar of PEG6000). Flax seeds was soaked by ethanol 70% for ten seconds and then washed with distilled water. Afterward, surface sterilaztion of seeds was done by using NaCl 0.2 % for 10 minutes and washed with distilled water. Surface sterilized seeds were treated by 150 ml of each strain culture over a two-hour period before sowing. 50 inoculated seeds were placed per petri plates and drought stress treatments exposed to each bacterial plates. Germination tests were accomplished in a dark growth chamber at 25±0.5°C and 75±1% of relative humidity. Seeds were considered germinated when radicles length were at least two mm. The number of germinated seeds was noted every day, and the final germination test was seven day. Germination percentage [21], germination rate [22] and the mean germination time [23] were calculated according to following relations, respectively:
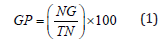
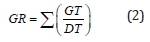
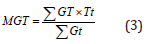
where, GP, NG, and TN represent germination percentage, total number of germinated seeds, and total number of seeds, respectively. GR is germination rate; GT stands number of germinated seeds in t days and DT denotes the days after sowing. MGT represent mean germination time and Tt denotes the time of Gt in days. Total radicle and plumule length were considered as seedlings height. For this purpose, ten plants were selected randomly and measured by the ruler. Radicle and plumule dry weight were measured after drying in oven at 70 ºC for 24 hours.
Pot Experiment
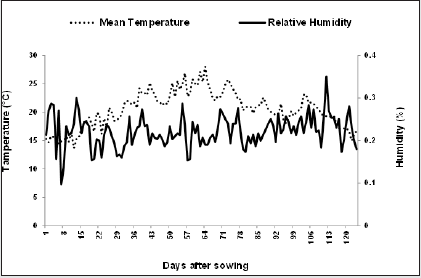
Complementary to the lab measurements, the pot experiments were conducted to investigate the bacterial effects more attentively, particularly when combined under controlled conditions. The experiment was carried out as factorial based on RCBD, with three replications on May 22, 2016, at the open area of research farm (2116 m above sea level; 32°21ʹN, 50°49ʹE) of Shahrekord University, Iran. Soil texture was clay loam with pH: 7.8, EC: 0.38 dS m-1, N: 0.11%, K: 470 mg kg-1 as K, and P: 17.6 mg kg-1 as P2O5. Daily temperature and humidity are presented in (Figure 1). The experimental factors included drought stress levels and bacterial strains. Irrigation contains three levels, 100%, 75%, and 50% crop water requirements designated as NDS (Non- Drought Stress), MDS (Moderate Drought Stress), and SDS (Severe Drought Stress), respectively; and bacterial inoculations include single, doublet, and triplet applications of the bacteria which had better result in the first test (C, B1, B2, A1, B1+B2, B1+A1, B2+A1, and B1+B2+A1). Pots with 20 cm diameter and 25 cm depth were filled with 4 kg soil. 15 seeds were planted in each pot and the seedlings were subsequently decreased to 10 after emergence. All the pots were kept in the open field. Growth period was 120 days (Figure 1).
Figure 1: Daily means temperature and relative humidity during the period from planting to harvesting (May 22 to August 22) in 2016 at Shahrekord University, Shahrekord, Iran.
Drought Stress Levels
The seedlings irrigated fully before applying the stress. Designed drought stress levels were initiated at the beginning of the stem elongation. Deficit irrigation was based on maximum allowable water depletion, i.e., MAD (%). To apply drought levels, a moisture meter (Delta-T, SM300, UK) was used to quantity the soil moisture content every second day and the plants were irrigated when the respective MAD threshold was reached. The irrigated water volume was calculated using the following relation:
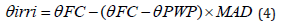
where, Ɵirri (%), ƟFC (%), and ƟPWP (%) represent root depth volumetric water content required by the crop, soil volumetric water content at FC (field capacity), and soil volumetric water content at PWP (permanent wilting point), respectively. The values obtained for ƟFC, ƟPWP (calculated by the volumetric method), and MAD were 34%, 18%, and 50%, respectively. The irrigation depth was determined based on the soil water moisture using following relations (5) and (6) [24]:
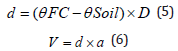
Where, d is irrigation depth (m), Ɵsoil denotes soil volumetric water content prior to irrigation (%), D represents pot depth (m), V is the irrigation volume applied (m3), and A labels pot area (m2). The experimental pots were irrigated by means of a graduated cylinder nearly every second day. Emergence percentage, emergence rate and the mean emergence time were calculated like laboratory test (Relation 1, 2, and 3). Samples to measure leaf dry weight and capsulated branches were taken between 11 a.m. and 14 p.m., when the plants in each treatment were at 50% their flowering stage (approximately 50-60 days after the planting date). For this purpose, three plants from each pot were randomly pulled out. Leaves were dried in a hot air oven for one day at 75°C. The number of capsulated branches was obtained from the average of plants. When the plants reached physiological maturity, samples were taken to determine biological yields. Samplings were done for plants that had not been used for prior measurements. Biological yield was obtained from the total dry weight of plants (grain+straw). At the end of the growing season, roots were washed, and all attached soils were removed. Root drying was performed similar to leaf drying. Root volume was measured with the use of graduated cylinder containing water according to Archimedes law [25].
Statistical Analysis
Variance analysis was carried out to compare the effects of different drought stress levels and bacterial treatments on measured traits using the SAS software. Mean comparisons were accomplished using the least significant difference (LSD) test (P<0.05).
Results
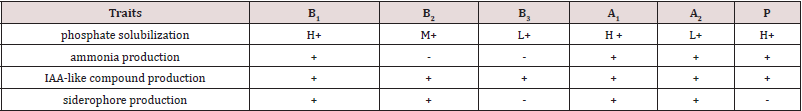
Bacterial ammonia, indole acetic acid, and siderophore production Growth contributed features of used bacterial strains are displayed in (Table 1). All the strains solubilize phosphate, produce ammonia (except for B2 and B3), IAA-like compounds, and siderophore (except B3 and P) (Table 1).
Table 1: Growth promoting features of bacterial strains (+: positive activity, -: no activity, H: high, M: medium and L; low phosphate solubilization ability).
Germination Attributes Traits Under Laboratory Experiment
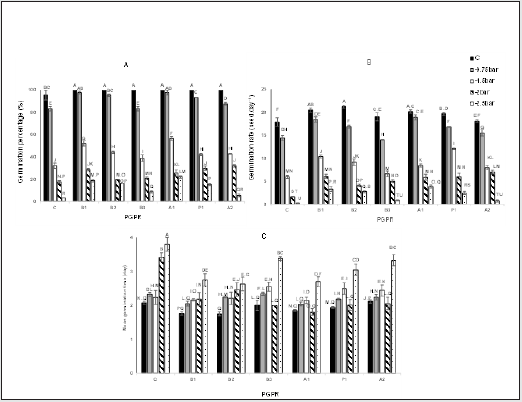
Percentage, rate, and mean time of germination were negatively affected by drought stress while bacterially inoculated treatments improved the mentioned traits (Figure 2). The highest germination percentage was observed for all inoculated seeds under C conditions. Considering severe stress or -2.5 bar of PEG, similarly, A1 and B2 had the highest percentage, with 5.7 and 4.7%%, respectively, in comparison with C treatments under the same stress level (Figure2-A). The highest germination rate (with a rise of 18.6.3%) was recorded in B1 and B2 treated seed under non-stress conditions relative to that recorded for C. The B1, B2 and A1 treatments under -2.5 bar PEG presented a significant increase in their rate, compared with C (Figure 2-B). The highest mean germination time was observed in the non-inoculated seeds under severe stress conditions (-2.5 bar PEG). However, significant difference did not observe between control plants with B1, B2, B3, A1, and P1 treatments under C conditions (Figure 2C, Figure 2).
Figure 2: Effects of drought stress and bacterial inoculation on germination percentage (A), germination rate (B), and mean germination time (C) under laboratory experiments. C: control; B1: B. amyloliquefaciens; B2: Bacillus sp. strain1; B3: Bacillus sp. strain2; A1: A. chroococcum; P: P. putida; A2: A. lipoferum.
Seedling’s Length and Dry Weight Under Laboratory Experiment
Non-stressed and bacterially inoculated treatments had higher length. The B1 seedlings exposed to normal non-stressed conditions had the highest length, exhibiting an increase of 18.3% relative to the height of the C seedlings. This is while the B2 treatments subjected to -2.5 bar PEG demonstrated even greater values, which were by 118.2% higher than those of C (Table 3-A). All bacterially inoculated seedlings had higher dry weight in comparisons with C ones, even though under stress condition the effect of each bacterial strain was different. The highest seedlings dry weight was observed in all bacterial treatments under non-stressed conditions. The B2 and P treatments subjected to -2. 5 bar PEG recorded the greatest weight, exhibiting an increase of 144.3% relative to the weight of the C seedlings (Table 3-B) (Figure-3).
Figure 3: Effects of drought stress and bacterial inoculation on seedlings length (A) and dry weight (B) under laboratory experiments. C: control; B1: B. amyloliquefaciens; B2: Bacillus sp. strain1; B3: Bacillus sp. strain2; A1: A. chroococcum; P: P. putida; A2: A. lipoferum.

Emergence Attributes Traits Under Pot Experiment
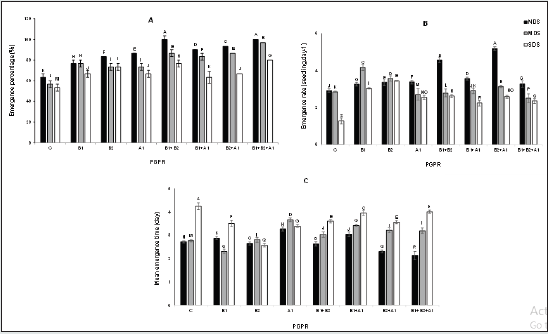
In pot experiments, the highest emergence percentage was recorded for B1+B2+A1 under NDS, showing an increase of 58%, compared with control NDS plants. Furthermore, B1+B2+A1 under MDS and SDS displayed increased emergence percentage compared to C plants (Figure 4-A). The plants inoculated with B2+A1 exposed to NDS, showed the highest germination rate by 78% higher than that of the C plants. B1 under MDS and B2 under SDS led to germination rate by 50% and 161.5% respectively, higher than what recorded for C plants in each stress levels (Figure 4-B). Non-inoculated plants under SDS showed mean emergence time by 57.4% higher than that of the C plants subjected to NDS. This is while the B1+B2+A1 plants under NDS conditions showed a reduction of 28.6% in time emergence when compared with those in C plants subjected to NDS (Figure 4-C).
Figure 4: Effects of drought stress and bacterial inoculation on emergence percentage (A), emergence rate (B), and emergence mean time (C) under pot experiments. C: control; B1: B. amyloliquefaciens; B2: Bacillus sp. strain1; A1: A. chroococcum.
Number of Capsulated Branches, Leaves Dry Weight, and Biological Yield
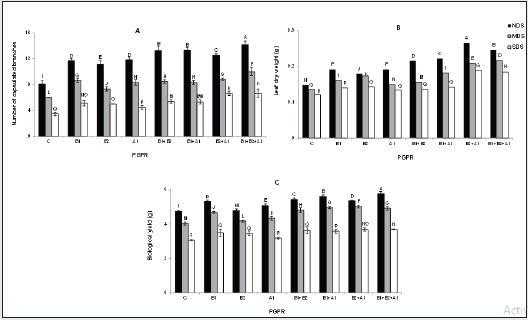
The number of capsulated branches was significantly higher in plants exposed to both non-deficit irrigation and bacterial inoculation. The number of capsulated branches in B1+B2+A1 plants subjected to NDS was 74.9% higher than the number of branches in control NDS plants. A similar result was obtained from MDS conditions. The B1+B2+A1 and B2+A1 plants exposed to SDS conditions had the highest capsulated branches number, showing an increase of 90.6% compared with those recorded for control plants under the same irrigation regimes (Figure 5-A). Under the pot experiment, higher leaf dry weight was recorded in bacterial inoculated plants with combined strains in comparison with those inoculated with individual ones. The highest weight was observed for the B2+A1 plants under NDS, showing an increase of 80.3% relative to that of C plants. Furthermore, the B1+B2+A1 treatments under MDS and SDS conditions revealed increased dry weight by 60% and 52.9%, respectively higher than the C treatments (Figure 5-B). Water deficit stress was observed to reduce biological yield in all the plants. The bacterially inoculated treatments exhibited increased biomass production, compared with those observed in the C plants. The plants inoculated with B1+B2+A1 showed the highest yield, i.e., 21.6% higher than C ones. This same inoculation treatment under SDS led to a yield that is 22.6% higher than those recorded for control treatments (Figure 5-C).
Figure 5: Effects of drought stress and bacterial inoculation on number of capsulated branches (A), leaf dry weight (B), and biological yield (C) under pot experiments. C: control; B1: B. amyloliquefaciens; B2: Bacillus sp. strain1; A1: A. chroococcum.
Roots Volume and Dry Weight
Fully irrigated and bacterially inoculated plants had maximum roots volume and dry weight. B1+B2+A1 treatments exposed to NDS conditions had 40% more volume compared to control NDS ones. Furthermore, B2+A1 treatments subjected to SDS had 85.6% more volume than C plants under SDS conditions (Table 2). The highest roots dry weight was observed for B1+B2+A1 ones under NDS conditions, showing an increase of 11.5%, compared to C plants. Similarly, the dry weight in this treatment under SDS recorded 13.3% increase, in comparison with untreated or C treatments under SDS conditions (Table 2).
Discussion
PGPRs use a variety of mechanisms to promote plant growth [26].
In our research, all the strains solubilized phosphate in the in vitro
assays nonetheless varied in the degree of solubilization ability. The
Bacillus and Pseudomonas genera are the most influential bacteria
that typically solubilize phosphate by producing organic acids
[27]. All the strains produced ammonia (except B2 and B3) which
considers as nitrogen source used for the macromolecule synthesis
in plants [28]. Nitrogen accessibility through bacteria can have
substantial role in providing other nutrients for yield production.
All six bacteria confirmed IAA-like compounds production.
According to reports, 80% of root-associated bacteria have the
ability for IAA secrete [29]. Moreover, all the bacteria (except B3
and P) created siderophore. Siderophore are low molecular weight
were secreted to solubilize iron from the surrounding environment
of the plants and to form a ferric-siderophore complexes, which
can be absorbed by plants [30]. Germination and emergence are
considered as important agronomic performance indices. In time
germination and uniform establishment of seedling has key impact
on crop’s final production. Content of soil water is an important
factor affecting seed germination and establishment. In the current
examination, PGPR inoculation has positive effects on germination
and emergence related traits of flaxseed under drought stress
conditions. The improved germination and emergence features
of inoculated seeds might be attributed to the bacterial growth
promoting properties, especially IAA production (Table 1). IAA is
an important phytohormone for plants growth [31]. Bacterial IAA
may increase plants endogenous IAA levels by increasing water uptake, photosynthesis, and IAA-related gene expression [32,33].
Increased seed germination of bacterial inoculated treatments may
be related to activity of some enzymes like hydrolytic enzymes
[34]. [35] reported that PGPRs through gibberellin production
may increase α-amylase activity and starch hydrolysis; therefore,
cytoplasmic membrane permeability will increase. At which point
minerals transition to embryo and germination will improve [35].
Moreover, in the pot experiment of current research, maximum
percentage and rate and minimum time emergence of triply- and
doubly inoculated treatments could be due to the synergistic effects
of the bacteria in mixed form than their individual application.
Application of Bacillus subtilis and Aospirillium brasilense and their
combination improved seed germination, seedling vigor index,
and promptness index of wheat under osmotic stress induced by
PEG6000 [36]. In their research with Bromus Tomentellus Bioss,
[34] found that, compared to the non-inoculated plants, under
drought stress, Azotobacter vinelandii, and Azotobacter vinelandii+
P.putida+Pantoea agglomerans at 0.7 FC level showed increased
seed germination rate. Studying Brassica napus, [37] reported
positive relations between seed bacteria and the mean germination
time of seedlings. [38] reported that the shortest germination time
of mature pepper seed was recorded in Bacillus strain inoculated
treatment while the longest mean germination time was exhibited
in the untreated control ones.
According to [39] report, seedlings length is a significant
index of crops agronomic performance. The non-inoculated
control plants in our study were more affected by drought stress,
whereas the bacterially inoculated treatments demonstrated
alleviated stress effects. Higher length recorded in inoculated
seedlings and plants might be associated with growth promoting
properties, particularly IAA production (Table 1). These hormones
are responsible for cell division and elongation, consequently,
increase plants length. IAA is closely related to growth promotion,
not only through embryogenesis, organogenesis, and vascular
differentiation but also via root and shoot development [40].
Gibberellins is also causing plant growth and development, seed
germination stimulation, flowering inhibition, dormancy breaking,
and root formation [41]. [42] reported that in Helianthus annus
shoot and root length significantly increased in inoculated PGPR
treatments under water stress. In their experiment with mexican
fir tree species, [43] found that, compared to the non-inoculated
plants, hydroprimed and inoculated treatments with Pseudomonas
and Bacillus strains had higher length. Water deficit leads to
stomatal closure and reduce the water content of aerial organs that
consequently decreases the number of capsulated branches. The
increased capsulated branches recorded in the treated flax plants
may be as a consequence of mitigated drought conditions through
the PGPR possible acting through phytohormones such as IAA
which promotes root and shoots development. Alterations in the
root morphology of PGPR treated plants could affect the number of
stems, shoots and leaves by facilitating the transmission of water
and nutrients toward aerial organs [44] It has been reported that
Agrobacterium tumefaciens and Rhizobium leguminosarum in faba
bean acted as PGPR increasing the number of pods and crop yield
[45].
Water deficit may through stomata closing or root growth
decreeing, lead plants water content reduction and consequently
causes dropping leaf growth and final biomass production.
The increased leaves dry weight and biological yield detected
in inoculated flax plants might be associated with the growth
promoting traits of used PGPRs (Table 1) [46]. reported that
applying PGPR to maize plants increased dry biomass of whole plant
parts. [47,48] attributed the increased in dry biomass of inoculated
plants to abilities like phosphate solubilization, production of root
promoting hormones namely IAA, cytokinins and gibberellin. In
[49] research on onion crops and bulbs, higher dry matter, yield
and dry weight were observed in bacterial inoculated treatments.
On the other hand, increased final yield in inoculated treatments
may be attributed to their on-time and uniform emergence and
establishment (Figure 2) which are crucial factors, and essential
to final biomass production and involve in complex phenomenon
of physiological and biochemical process. Agroecosystems can
be contributed with plant growth promoting rhizobacteria
mediated root trait changes through improving crop stand,
resource use efficiency, stress tolerance, and soil structure. PGPRs
by phytohormones, volatile organic complexes, and secondary
metabolites production can affect plants root features, subsequent
improved nutrient exchange and rhizosphere effects [50]. The effect
of PGPRs on root length, biomass and volume have been reported in
several experiments [51-53].[54] maintained that Bradyrhizobium
japonicum and Pseudomonas putida in soybean crops improved
root length, surface area, root volume, nodules number mainly due
to the bacterially IAA production and phosphorous and nitrogen
accumulation. In peanut, the improved root dry weight and
root length reportedly belonged to plants receiving inoculation
of Bacillus sp., Pseudomonas sp., and co-inoculation with
Bradyrhizobium sp. by IAA production, antagonistic against root
disease, biofilm formation, and lytic enzyme production [55].
Conclusion
Drought was found to have undesirable influences on percentage, rate, and mean time of germination, seedlings dry weight and height in the laboratory experiment and the percentage, rate, and mean time of emergence, number of capsulated branches, leaves dry weight, biological yield, root volume and dry weight in the pot part. However bacterial inoculation was detected to decline the adverse effects of unfavorable conditions. The results indicated that investigated characters varied with different bacterial strains significantly (P ˂ 0.05). The B1 strain in the laboratory part and B1+B2+A1 treatment in the pot experiment had better results in comparison with other ones. The observed improvement measured traits may be attributed to the phosphate solubilization, ammonia, IAA-like compounds, and siderophore production of the bacteria used and their synergistic effects when used as a combination. Briefly, PGPR application possibly will be a promising technique to increase drought stress tolerance in oilseed crops such as flax.
References
- Tavarini, S, Castagna A, Conte G, Foschi L, Sanmartin C, et al. (2019) Evaluation of chemical composition of two linseed varieties as sources of health-beneficial substances. Molecules 24(20): 3729.
- Sanmartin C, Taglieri I, Venturi F, Macaluso M Zinnai A, et al. (2020) Flaxseed cake as a tool for the improvement of nutraceutical and sensorial features of sourdough bread. Foods 9(2): 204.
- Kiryluk A, Kostecka J (2020) Pro-environmental and health-promoting grounds for restitution of flax (Linum usitatissimum L) cultivation. Journal of Ecological Engineering 21(7): 99-107.
- Khan N, Zandi P, Ali S, Mehmood A, Adnan M, et al. (2018) Impact of salicylic acid and PGPR on the drought tolerance and phytoremediation potential of Helianthus annus. Frontiers in microbiology 9: 2507.
- Marthandan V, Geetha R, Kumutha K, Renganathan VG, Karthikeyan A, et al. (2020) Seed priming: A feasible strategy to enhance drought tolerance in crop plants. International Journal of Molecular Sciences 21(21): 8258.
- Kawasaki T, Akiba T, Moritsgu M (1983) Effects of high concentrations of sodium chloride and polyethylene glycol on the growth and ion absorption in plants. I Water culture experiments in a green house Plant & Soil 75: 75-85.
- Rajabi Khamseh S, Shekari F, Zangani E (2013) The effects of priming with salicylic acid on resistance to osmotic stress in germination stage of wheat. International Journal of Agriculture 3: 543
- Liu M, Li M, Liu K, Sui N (2015) Effects of drought stress on seed germination and seedling growth of different maize varieties. Journal of Agricultural Science 7(5): 231-240.
- Silva de Almeida JA, Guedes de Azevedo MTVL, Salomon MV, Median PF (2018) Water stress in germination, growth and development of coffee cultivars. Journal of Seed Science 40(1): 82-89.
- Queiroz MS, Oliveira CE, Steiner F, Zuffo AM, Zoz T, et al. (2019) Drought stresses on seed germination and early growth of maize and sorghum. Journal of Agricultural Science 11(2): 310-318.
- Pavli OI, Foti C, Skoufogianni G, Karastergiou G, Panagou A, et al. (2020) PEG-Induced drought stress during germination effects on soybean germplasm. Agricultural Research & Technology 23(4): 70-80.
- Marthandan V, Geetha R, Kumutha K, Renganathan VG, Karthikeyan A, et al. (2020) Seed priming: A feasible strategy to enhance drought tolerance in crop plants. International Journal of Molecular Sciences 21(21): 8258.
- Mahmood A, Turgay OC, Farooq M, Hayat R (2016) Seed biopriming with plant growth promoting rhizobacteria: a review. FEMS Microbiology Ecology 92(8): fiw112.
- Sansinenea E (2019) Bacillus spp: As plant growth-promoting bacteria: 225- 237 In: Secondary metabolites of plant growth promoting rhizomicroorganisms. Singh H, Keswani C, Reddy M, Sansineea E, Garcia-Estrada C (eds) Springer Nature Singapore.
- Verma DK, Pandey AK, Mohapatra B, Srivastava S, Kumar V, et al. (2019) Plant growth-promoting rhizobacteria: An eco-friendly approach for sustainable agriculture and improved crop production: 3-80.
- Ilyas N, Mumtaz K, Akhtar N, Yasmin H, Sayyed RZ, et al. (2020) Exopolysaccharides producing bacteria for the amelioration of drought stress in wheat. Sustainability 12(21): 8876.
- Tewari S, Arora NK (2014) Multifunctional exopolysaccharides from Pseudomonas aeruginosa PF23 involved in plant growth stimulation, biocontrol and stress amelioration in sunflower under saline conditions. Current microbiology 69(4): 484-494.
- Goswami D, Dhandhulia P, Patel P, Thakker JN (2014) Screening of PGPR from saline desert of Kutch: Growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiological Research 169(1): 66-75.
- Glickmann E, Dessaux Y (1995) A critical examination of the specificity of the Salkowski reagent for indolic ompounds produced by phytopathogenic bacteria. Applied & Environmental Microbiology 61(2): 793- 796.
- Hu QP, Xu JG (2011) A simple double-layered chrome azurol S agar (SDCASA) plate assay to optimize the production of siderophores by a potential biocontrol agent Bacillus. African Journal of Microbiology Research 5(25): 4321-4327.
- Louden BC, Haarmann D, Lynne AM (2011) Use of blue agar CAS assay for siderophore detection. Journal of Microbiology & Biology Education 12(1): 51-53.
- Ikic I, Maricevic M, Tomasovic S, Gunjaca J, Atovic ZS, et al. (2012) The effect of germination temperature on seed dormancy in Croatian-grown winter wheat Euphytica 188: 25-34.
- Kalsa KK, Abebie B (2012) Influence of seed priming on seed germination and vigor traits of Vicia villosa ssp dasycarpa (Ten). African Journal of Agricultural Research 7(21): 3202-3208.
- Kaya YK, Arisoy RZ, Gocmen A (2002) Variation in grain yield and quality traits of bread wheat genotypes by zinc fertilization. Journal of Agronomy1(3): 142-144.
- Taherpazir S, Hashemabadi D (2016) The Effect of cycocel and pot size on vegetative growth and flowering of Zinnia (Zinnia elegans). Journal of Ornamental Plants 6: 107-114.
- Figueiredo MVB, Bonifacio A, Rodrigues AC, Araujo FF (2016) Plant growth-promoting rhizobacteria: key mechanisms of action: 23-37.
- Rodríguez H, Fraga R, Gonzalez T, Bashan Y (2006) Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant & Soil 287: 15-21.
- Hayat R, Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion: A review. Annals of Microbiology 60: 579-598.
- Patten CL, Glick BR (1996) Bacterial biosynthesis of indole-3-acetic acid. Canadian Journal of Microbiology 42(3): 207-220.
- Andrews SC, Robinson AK, Rodríguez-Quiñones (2003) Bacterial iron homeostasis. FEMS Microbiology Reviews 27(2-3): 215- 237.
- Khan AL, Waqas M, Kang SM (2014) Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. Journal of Microbiology 52(8): 689-695.
- Etesami H, Alikhani HA, Mirseyed Hosseini H (2015) Indole-3-acetic acid and 1-aminocyclopropane-1-carboxylate deaminase: Bacterial traits required in rhizosphere, rhizoplane and/or endophytic competence by beneficial bacteria:183- 258.
- Tsukanova KA, Meyer JJM, Bibikova TN (2017) Effect of plant growth-promoting rhizobacteria on plant hormone homeostasis. South African Journal of Botany 113: 91-102.
- Delshadi S, Ebrahimi M, Shirmohammadi E (2017) Effectiveness of plant growth promoting rhizobacteria on Bromus tomentellus Boiss seed germination, growth and nutrients uptake under drought stress. South African Journal of Botany 113: 11-18.
- Zahir ZA, Arshad M, Frankenberger WT (2004) Plant growth promoting rhizobacteria: applications and perspectives in agriculture. Advances in Agronomy 81: 98-169.
- Ilyas N, Mumtaz K, Akhtar N, Yasmin H, Sayyed RZ, et al. (2020) Exopolysaccharides producing bacteria for the amelioration of drought stress in wheat. Sustainability 12(21): 8876.2
- Rochefort A, Briand M, Marais C, Wagner MH, Laperche A, et al. (2019) Influence of environment and host plant genotype on the structure and diversity of the Brassica napus seed microbiota. Phytobiomes Journal 3(4): 326-336.
- Yildirim KC, Canik Orel D, Okyay H, Gursan MM, Demir I (2021) Quality of immature and mature Pepper (Capsicum annuum L) seeds in relation to bio-priming with endophytic Pseudomonas and Bacillus spp. Horticulturae 7(4): 75.
- Nadeem M, Pham TH, Thomas R, Galagendara L, Lavanagh V, et al. (2019) Potential role of root membrane phosphatidic acid in superior agronomic performance of silage-corn cultivated in cool climate cropping systems. Physiologia Plantarum 167(4): 1-12.
- Etchells JP, Smith ME, Gaudinier A, Williams CJ, Bradly SM (2016) A brief history of the TDIF-PXY signaling module balancing meristem identity and differentiation during vascular development. New Phytologist 209(2): 474-484.
- Gupta R, Chakrabarty SK (2013) Gibberellic acid in plant: still a mystery unresolved. Plant signaling & behavior 8(9): 25504.
- Khan N, Zandi P, Ali S, Mehmood A, Adnan M, et al. (2018). Impact of salicylic acid and PGPR on the drought tolerance and phytoremediation potential of Helianthus annus. Frontiers in microbiology 9: 2507.
- Zulueta-Rodríguez R, Hernández-Montiel LG, Murillo-Amador B, Rueda-Puente EO, Capistrán LL, et al. (2015) Effect of hydropriming and biopriming on seed germination and growth of two Mexican fir tree species in danger of extinction. Forests 6(9): 3109-3122.
- Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, et al. (2012) Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytologyst 193(1): 30-50.
- Youseif SH, El-Megeed A, Fayrouz H, Saleh SA (2017) Improvement of faba bean yield using Rhizobium/Agrobacterium inoculant in low-fertility sandy soil. Agronomy 7(1): 2-12.
- Kuan KB, Othman R, Abdul Rahim K, Shamsuddin ZH (2016) Plant growth-promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation and nitrogen remobilization of maize under greenhouse conditions 11(3): 0152478.
- Wu S, Cao ZH, Li ZG, Cheung KC, Wong MH (2005) Effects of biofertilizer containing N-fixer, P and K solubilizer and AM fungi on maize growth: a greenhouse trial. Geoderma 125(1-2): 155-166.
- Montañez A, Abreu C, Gill PR, Hardarson G, Sicardi M (2009) Biological nitrogen fixation in maize (Zea mays L) by 15 N isotope-dilution and identification of associated culturable diazotrophs. Biology & Fertility of Soils 45(3): 253-263.
- Pellegrini M, Spera DM, Ercole C, Del Gallo M (2021) Allium cepa L inoculation with a consortium of plant growth-promoting bacteria: Effects on plants, soil, and the autochthonous microbial community. Microorganisms 9(3): 639.
- Grover M, Bodhankar S, Sharma A, Sharma P, Singh J, et al. (2021) PGPR mediated alterations in root traits: way towards sustainable crop production. Frontiers in Sustainable Food Systems 4: 287.
- Schwartz A, Ortiz I, Maymon M, Herbold C, Fujishige N, et al. (2013) Bacillus simplex-A little known PGPB with anti-fungal activity-alters pea legume root architecture and nodule morphology when coinoculated with Rhizobium leguminosarum bv. viciae. Agronomy 3(4): 595-620.
- Korir H, Mungai NW, Thuita M, Hamba Y, Masso C (2017) Coinoculation effect of Rhizobia and plant growth promoting Rhizobacteria on common bean growth in a low phosphorus soil. Frontiers in Plant Science 8: 141.
- Mesa-Marín J, Del-Saz NF, Rodríguez-Llorente ID, Redondo-Gómez S, Pajuelo E et al. (2018) PGPR reduce root respiration and oxidative stress enhancing Spartina maritima root growth and heavy metal rhizoaccumulation. Frontiers in plant science 9: 1500.
- Egamberdieva D, Wirth S, Jabborova D, Räsänen LA, Liao H (2017) Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture. Journal of Plant Interactions 12: 100- 107.
- Yuttavanichakul W, Lawongsa P, Wongkaew S, Teaumroong N, Boonkerd N, et al. (2012) Improvement of peanut rhizobial inoculant by incorporation of plant growth promoting rhizobacteria (PGPR) as biocontrol against the seed borne fungus, Aspergillus niger. Biological Control 63(2): 87-97.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...





