Lupine Publishers Group
Lupine Publishers
Menu
Research Article(ISSN: 2641-6875) 
Assessment and Mapping of Intestinal Parasitic Infections and Associated Risk Factors from Different Northern Health Centers in Rwanda Volume 2 - Issue 2
Cedrick Izere1 and GR Neel2*
- 1Assistant Lecturer, Department of Biomedical Laboratory Sciences, Rwanda
- 2Associate Professor, Department of Biotechnology, Rwanda
Received: November 19, 2020; Published: December 18, 2020
*Corresponding author: Roua Lajnaf, Alimentary Analysis Unit, National Engineering School of Sfax, BPW 3038, Sfax, Tunisia
DOI: 10.32474/CTBM.2020.02.000133
Abstract
Aim: The general objective of this study involved the assessment and mapping of intestinal parasitic infections in Rwanda.
Methods: In this study, Rwandan health centers and hospitals from different region were targeted. For each suspected
patient was given a labeled stool container to collect stool sample. The stool samples were carried to parasitological laboratory
for parasitological examination. Macroscopic examination was performed, and direct smears prepared with normal saline and/
or iodine for parasites analysis under microscope (objectives 10×and 40×). Risk factors associated to intestinal parasites were
assessed using structured questionnaires given to patients. Obtained cross sectional results of intestinal parasites prevalence and
associated risk factors from different Rwandan Northern health centers were used, analyzed by SSPSS version 16, Microsoft Excel
and Arc Map and records are incorporated into a geographical system for mapping.
Results: The overall prevalence of intestinal parasitic infections in Rwanda according to 3139 patients who answered well the
questionnaire was 57% among the age group below 5 years old, 54.96% among the age group of 5-15 years old, 57.41% among the
age group of 15-25 years old, 55.37% among the age group of 25-35 years old, 59.59% among the age group of 35-45 years old and
49.40% among the age group of ≤45 years old. In the present study, the prevalence of intestinal parasitic infections was significantly
65.39% among the age group of ≥45 years and was higher compare to other age groups.
Conclusion: This current study has tried to point out relatively assessment and mapping of intestinal parasitic infections and
associated risk factors in Rwanda. The relatively high prevalence rate of intestinal parasites infections in Rwanda are the reflection
of poor sanitation of the environment, poor personal hygiene, relatively unhygienic water supply and lack of clean drinking water
supply. Those were the main risk factors for intestinal parasites infection in Rwanda.
Recommendation: There is a need for community mobilization towards provision and use of safe and adequate water supply,
latrine construction to reduce open field defecation. The high overall prevalence of intestinal parasitic infections in this present
study need the mass deworming in the community and establishment of good personal hygiene and environmental sanitation;
public health education is also necessary on the transmission of intestinal parasitic infections in communities, participatory
approaches and combined efforts from the community and health sectors are needed to control the study areas.
Keywords: Protozoans; Helminthes; Intestinal Parasitic Infections
Introduction
A parasite is an organism that lives on or in a host organism and gets its food from or at the expense of its host. There are three main classes of parasites that can cause disease in humans: protozoa, helminths, and ectoparasites (CDC, 2017). Over half of the world’s population is infected with eukaryotic pathogens. Parasitic infections, caused by intestinal helminths and protozoan parasites, are among the most prevalent infections in humans in developing countries [1]. In developed countries, protozoan parasites more commonly cause gastrointestinal infections compared to helminths. Intestinal parasites cause a significant morbidity and mortality in endemic countries. The World Health Organization (WHO) ranks six parasitic diseases among the top 20 microbial causes of death in the world. Every year, there are over 5 million new cases of malaria, schistosomiasis, amoebiasis, hookworm, African trypanosomiasis and intestinal parasites reported in developing countries. According to Mama (2016) it is estimated that one third of Ethiopians are infected with Ascaris lumbricoides [2], one quarter is infected with Trichuris trichiura and one in eight lives with hookworm. As a result, Ethiopia has the second highest burden of ascariasis, the third highest burden of hookworm, and the fourth highest burden of trichuriasis in Sub-Saharan Africa. In Africa, more specifically Sub-Saharan Africa, parasitic infections are the major public health problem and most of the victims are children. Currently, the protozoan parasite (Entamoeba histolytica and Giardia intestinalis) and the soil transmitted helminthes (Ascaris lumbricoides, Trichuris trichiura, and Hookworm) are the leading intestinal parasites which cause significant morbidity and mortality in the Africa (Workneh et al., 2014). Intestinal parasitic infection has been estimated to be responsible for 25-75% of all childhood illnesses and episodes of intestinal parasitic to about 14% of outpatient visits and 16% of hospital admissions and accounted for an average of 35 days of illness per year in children aged less than five years (Merid et al., 2017). In Rwanda six species of intestinal helminthes with an overall prevalence of 65.8% [3] have been identified among school children 10-16 years old from 136 primary schools in 2008. The predominant parasites were A. lumbricoides observed in 38.6% of children, followed by A. G duodenal in 31.6% of the children. Another study performed in 2010 among children less than five years of age revealed that 20% and 60.1% [4] of them were infected by G. duodenalis using the microscopic. Intestinal parasitic infections are still a major public health problem, in Rwanda especially in Northern parts due to many different risk factors that appeared in that area like the use of stagnant water and untreated water and there are insufficient and closed latrines in the village surrounding these health centers [5].
Research Methodology
Study design
The study was to deal with the assessment and mapping of intestinal parasitic infections and associated risk factors from different Northern health centers in Rwanda that predispose people for intestinal parasitic infection [6]. It is a meta-analysis study that carried out from January 2016 to November 2017 on some health centers and hospitals in Rwanda.
Description of the study area
The present study was carried out in Rwanda [7] is located in central Africa, to the east of the Democratic Republic of the Congo, at the co-ordinates 2°00′S 30°0′E. At 26,338 square kilometers.
\Sample population
This study deals with 18512 patients suspected of intestinal parasites from January 2016 to November 2017 on some health centers and hospitals in Rwanda.
Sample size
The study was carried out on 3839 patients who attended northern health centers and hospitals in Rwanda, answered well the questionnaire and having diarrhoea with or without mucous or blood, fever, abdominal distinction, nausea, vomiting, anal itch, [8] abdominal pain and anemia symptoms in the period of the study from January 2016 to November 2017.
Methods of Data Collection
Collection of stool samples
This research project has been conducted based on different research projects conducted with different authors from INES Ruhengeri during 2016-2017. During all those studies, each individual was given a stool container to collect the sample [9]. The stool samples were carried to parasitological laboratory and this was done in each health center parasitological laboratory for parasitological examination.
Questionnaire Survey
The content of the questionnaire included full identification of the patient and risk factors that are associated to intestinal parasitic infections (water utilization and source, food hygiene body hygiene, toilet utilization and animals in the family) [10]. The questionnaire was originally developed in English and then translated into local language (Kinyarwanda). For children who were not able to answer to the questions on the questionnaire their parents or caretakers took the responsibility of filling the questionnaires. The questionnaire assessed hygienic habits in suspect patients of intestinal parasites such as socio-economic [11] status which provides information on social status (icyiciro cy’ubudehe), level of education, occupation, water consumed, food hygiene, body hygiene, toilet utilization and animals in the family (live with or handle animal in your family, kind of animal do you frequently handle). Questionnaires were given to patients after delivering stool samples.
Laboratory Parasitological Examination Procedures
Macroscopic examination of stool samples
All collected stool samples were characterized macroscopically based on their consistency (formed, lose or watery), presence and absence of larval stages of parasitic infections and other macroscopic features seen by the researcher in the presence of experienced laboratory technician [12]. Intestinal parasitic infections results were recorded, and risk factors assessed. The sample were tested macroscopically for checking if there is no other contamination and clearly see the color and their consistence.
Direct microscopy (wet mount)
A direct wet mount was prepared, stool samples mixed with normal saline and iodine and mounted on microscopic. Slides were then examined under light microscopy. Direct microscopic examination for ova, cysts and parasitic infections was carried out under the [13] supervision of experienced medical laboratory technician and diagnosis was made on the basis of morphology and size microscope at 10X and 40X magnifications.
Results and Discussion
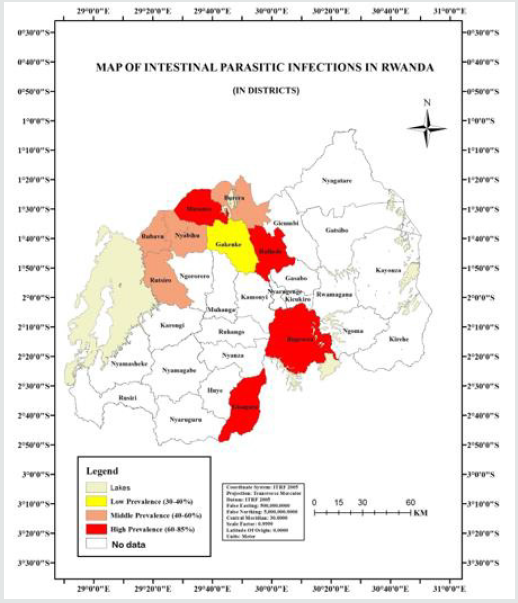
In Musanze district E. histolytica had high Figure 1 prevalence of 31.49%, followed by A. lumbricoides with 28.66%. As a big number of Musanze citizen are farmers, could be infected by the soil as A. lumbricoides is among the soil-transmitted helminths. Many mountain found in this area do not facilitate piping water to citizens of Musanze district [14]. Rural to urban migration rapidly increases the number of food eating places in towns and their environs. Some of these eating establishments have poor sanitation and are overcrowded, facilitating disease transmission as amoebiasis, especially through food-handling (Kamau et al. 2012) Figure 2. In Gakenke district E. histolytica had high prevalence of 39.24%, followed by E. coli with 31.85%. These two parasites are responsible for amoebiasis [15]. As cysts caused the disease are ingested through food or water or direct contact with fecal matter and can resist several months relatively inactive form in the soil or environment where the feces are deposited, these facts could increase the prevalence. As in this area there is high number of inadequate and lack of latrines. Same results found in Rwanda also in 2013, that took place in Rukara Health center where the overall prevalence was assessed to be [16] 56.1% among 193 cases tested where the high prevalence was found to be for Entamoebahistolytica followed by Entamoeba coli with a high number of infected persons observed in females than in males (Niyizurugero et al., 2013) Figure 3.
Figure 1: Prevalence of intestinal parasites in Musanze district. Shows the different intestinal parasites found in Musanze district.
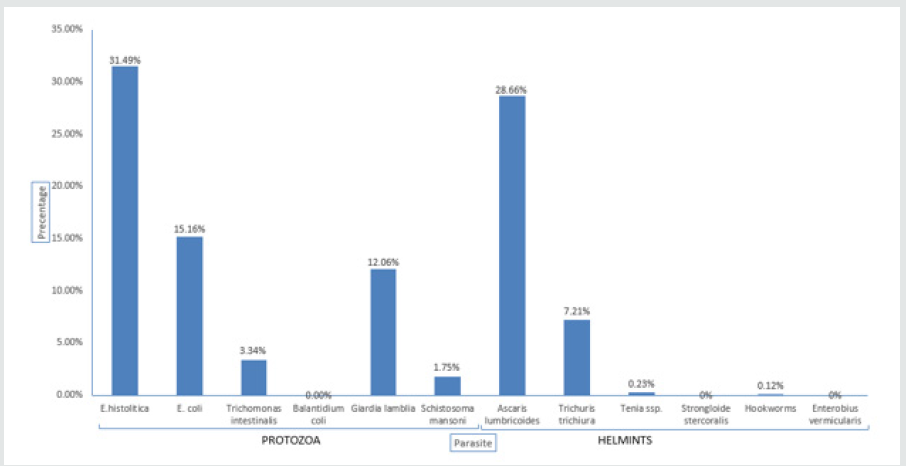
Figure 2: Prevalence of intestinal parasites in Gakenke district. Shows the different intestinal parasites found in Gakenke district.
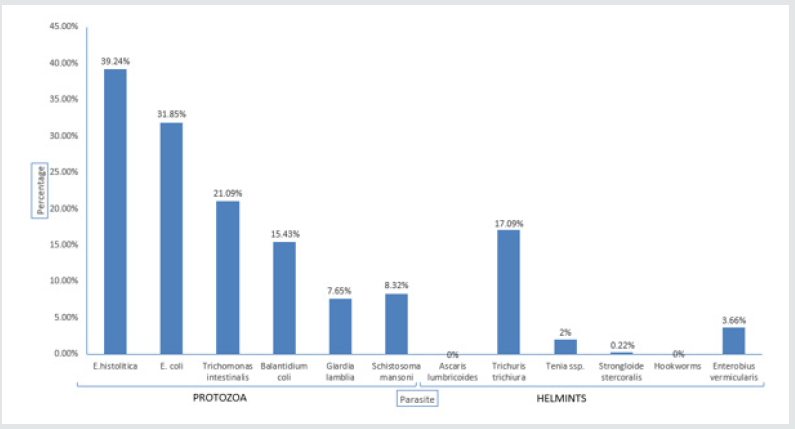
Figure 3: Prevalence of intestinal parasites in Nyabihu district. Shows the different intestinal parasites found in Nyabihu district.
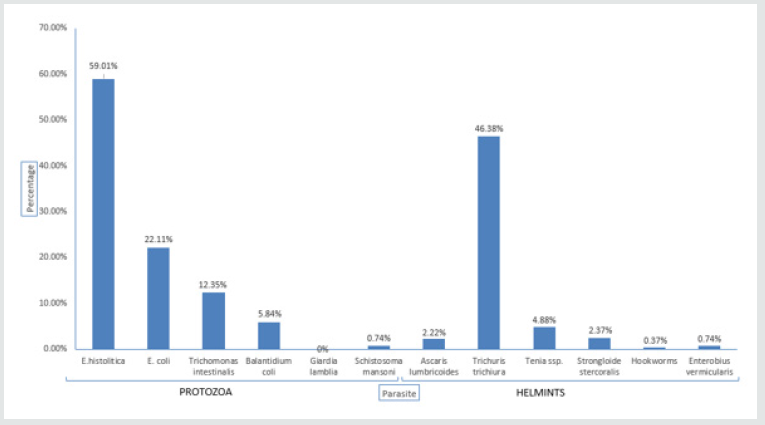
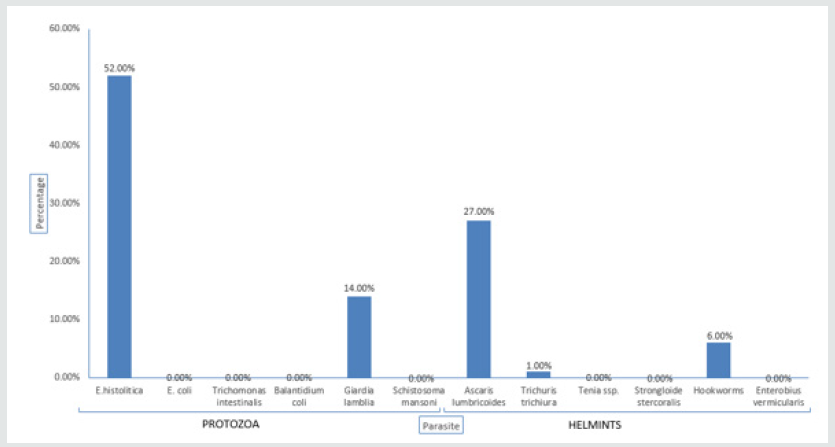
In Nyabihu district E. histolytica had high prevalence of [17] 59.01%, followed by Trichuris trichiura with 46.38%. The whipworm is especially prevalent in areas of high rainfall, high humidity and dense shade. 83% are farmers and animal keepers, they much time in the farms, where there is not at least pipe water. This increases poor food and body hygiene which increase the prevalence of parasitic infections in Nyabihu district. Same results showed that the prevalence of intestinal parasites diagnosed in the United States among African refugees [18], Variables included results of microscopy of a single stool specimen, age, sex, ethnicity and departure origin, were 1,254 refugees, 56% had intestinal parasites. 14% had helminths; In addition, 52% had protozoans as high prevalence. The most common pathogens were Giardia lamblia (14%) and Trichuris trichiura (9%) (Kassa, 2007) Figure 3. In Rulindo district A. lumbricoides had high prevalence of 41.90%, followed by E. histolytica with 20.42% Figure 4. The reason of this high prevalence is the same as those of Musanze district [19]. The highest prevalence of ascariasis occurs in tropical countries where warm, wet climates provide environmental conditions that favor year round transmission of infection which shows same results. This contrasts to the situation in dry areas where transmission is seasonal, occurring predominantly during the rainy months. The prevalence is also greatest in areas where suboptimal sanitation practices lead to increased contamination of soil and water more than 1.5 billion people or 24% [20] of the world’s population are infected with soil-transmitted helminth infections worldwide [21] Figure 5. In Burera district E. histolytica had high prevalence of 52%, followed by A. lumbricoides with 27% and Giardia lamblia with 14%. The reason of high prevalence is because there is not sufficient pipe water and, in this area, there is lakes. People use lake water as is nearby.
Figure 4: Prevalence of intestinal parasites in Rulindo district. Shows the different intestinal parasites found in Rulindo district.
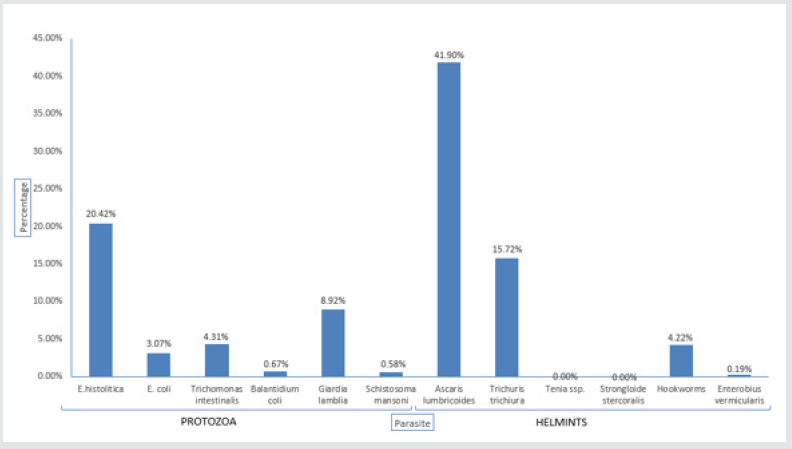
Figure 5: Prevalence of intestinal parasites in Burera district. Shows the different intestinal parasites found in Burera district.
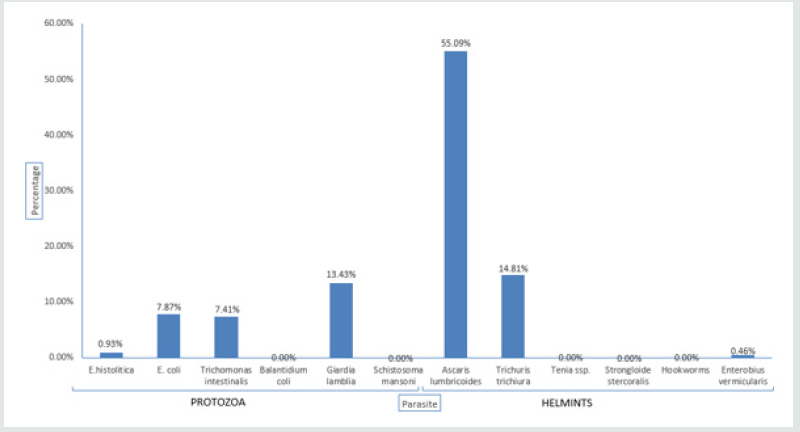
This is in agreements with the previous epidemiological studies in Nahavand County, western Iran. (Omorodion et al.,2012) demonstrated high prevalence of IPIs among different population and had shown that Entamoeba histolytica and Ascaris lumbricoides are the predominant IPIs [22]. Amoebiasis is the third leading cause of death from parasitic diseases worldwide, with its greatest impact on the people of developing countries (Lebbad et al., 2002). Practice of hygiene by washing vegetables and other food before cooking, use proper water and washing hands while preparing meals or eating are good measures of preventing amoebiasis infection [23] Figure 6. In district A. lumbricoides had high prevalence of 55.09%, followed by Trichuris trichiura with 14.81%. The reason of high prevalence is the same as Burera, there is not sufficient pipe water they prefer Lake Kivu. People use lake water as is nearby. This result is comparable with another study from Guatemala, 60% of the population in a rural village was infected with A. lumbricoides and 41% with T. trichiura [24]. Higher prevalence was found in the studies from the eastern part of Turkey, where the socio-economic and environmental conditions were lower. Infection rates are also high in temperate areas with poor sanitation. Most cases of amoebiasis in the [25] Unites States occur in immigrants from endemic areas, in HIV infected patients and people living in states that border Mexico (Stanley, 2003) [26].
Risk Factors and their Association of Intestinal Parasitic Infections
Table 3 [27] shows the relationship between intestinal parasitic infections and associated risks factors among patients attending health centers and hospitals in Rwanda [28]. Accordingly, the prevalence of intestinal parasitic infection was linked to socioeconomic status whereas these characteristics can play a major role in diseases contraction [29]. The high prevalence of intestinal parasites is closely related to low education, poverty, poor environmental condition lack of clean water as stated by [30]. The high observed percentage of intestinal parasites infection, according to risk factors, is caused by poor habitation, poor water utilization [31] (70%) does not use pipe water always, poor toilet use (70.1%) [32], not cleaning kitchen always (71%) and not cleaning kitchen materials with pipe water and detergent (80%) [33] are positive. In Table 1, except swimming in fresh water, lakes, steam and ponds which shows that there is no significant with association with intestinal parasitic infections. Other factors show that there is significance. We have to insist on those all factors which show the strong relationship with intestinal parasites. According to the results regarding to food hygiene our results agreed with those from Ethiopia [34] which showed that the fact of not cleaning kitchen materials with pipe water and detergent, not always cleaning the kitchen and not washing fresh fruits before eating had a statistically significant association (P<0.05) with the prevalence of intestinal parasitic infections [35]. All of three factors recorded a strong relationship with intestinal parasitic infections and current results agreed with results from Burkina Faso [36]. Same results with studies from Ethiopia (Mama & Alemu, 2016), [37]; Kenya (Muli et al., 2014); had a clear agreement to our results that showed that body hygiene in its some components (not washing hands with pipe water and detergent, not washing hands after toilet [38], not cutting fingernails after growing and swimming in fresh water, lakes, stream) showed a statistically significant and strong association with the prevalence of intestinal parasitic infections (P<0.05) [39].
Figure 6: Prevalence of intestinal parasites in Rubavu district. Shows the different intestinal parasites found in Rubavu district.
Mapping of intestinal parasitic infections
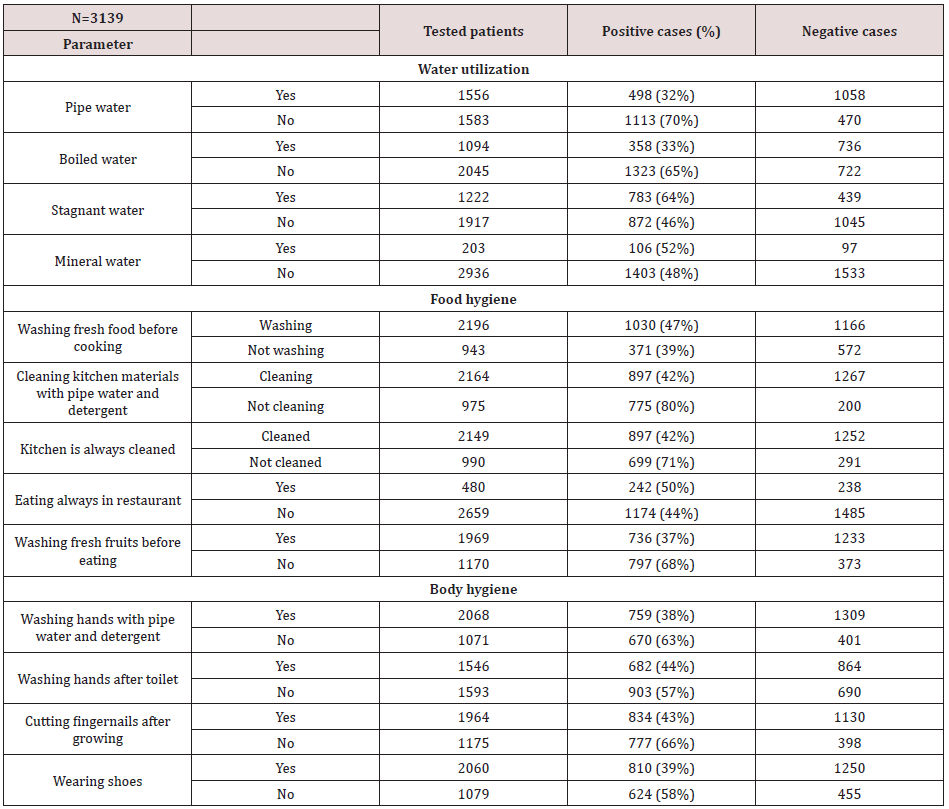
Intestinal parasitic infections are still a major public health problem [40], in Rwanda especially in that area due to many different risk factors that appeared in that area like the use of stagnant water and untreated water and there are insufficient and closed latrines in the village surrounding these health centers [41]. The Figure 7 shows map of intestinal parasitic infections in provinces and Figure 7 shows map of intestinal parasitic infections in districts [42]. Of the 3,828 children examined for intestinal parasitic infections, 1059 (27.66%) [43] were infected. Parasites identified in the study were Ascaris lumbricoides 51.78%, hookworm 25%, Trichuris trichiura 15.18%, Strongyloides stercoralis 7.14 %, taenia sp 0.89%, Enterobis vermicularis 0.01%. The Co-ordinates (latitude and longitude) obtained using the field Model GPS (Garmin GPS MAP76s Chart plotting receiver) were converted from degrees/minutes/seconds to decimal degrees giving the values for easting and northing. The values were displayed in Arc map/Arc info version 10.0 and this gave the location of the school’s rate of infections [44]. The geostatistical analyst wizard was employed using the Kriging method. Two data sets were used; number of positive/number examined x100. This was the determining factor. The value was then imputed into the geostatistical wizard and the map produced [45]. Figure 11 shows that, Burera had prevalence of 56.02%, Gakenke had 39.24%, Musanze had 60.01%, Gisagara had 72.58%, Bugesera had 60.77% Nyabihu had 59.01%, Rubavu had 48.00%, Rutsiro had 44.39%, Rulindo had high prevalence 80.29% of intestinal parasitic infections among other districts in Rwanda [46]. For instance, in a study to determine the Prevalence of intestinal parasite infections and associated risk factors among students at Kigali Institute of education in Rwanda, more than 50.5% of the stools were found to be infected with an intestinal parasite E. histolytica was the highest with 54.5%, Trichomonas intestinalis and Ascaris lumbricoides were 20.0%, Giardia 3.6% and A. duodenale 1.8%. The global statistics results are summarized in Table 1. Factors like not using pipe and boiled. Usage of stagnant water, not cleaning kitchen materials with pipe water and detergent, not always cleaning the kitchen and not washing fresh fruits before eating; not washing hands with pipe water and detergent, not washing hands after toilet, not cutting fingernails, growing and swimming in fresh water, lakes, stream and not using toilet were statistically significantly associated with intestinal parasitic infections (P<0.05).
Conclusion
This current study has tried to point out relatively assessment and mapping of intestinal parasitic infections and associated risk factors in Rwanda. The relatively high prevalence rate of intestinal parasites infections in Rwanda are the reflection of poor sanitation of the environment, poor personal hygiene, relatively unhygienic water supply and lack of clean drinking water supply. Those were the main risk factors for intestinal parasites infection in Rwanda. Eastern and southern province had high prevalence in interval of 60 and 85%. E. histolytica has highest prevalence of intestinal parasites in Rwanda with 30.10%. In districts, E. histolytica has highest prevalence of intestinal parasites also, with 31.49% in Musanze district, 44.86% in Rutsiro, 39.24% in Gakenke, 59.01% in Nyabihu, 52.00% in Burera, 60.77% in Bugesera, 72.38% in Gisagara and Ascaris lumbricoides has highest prevalence of intestinal parasites, with 41.90% in Rulindo and 55.09% in Rubavu. Statistical results showed that risk factors prevalence, of poor water utilization was (56.97%), poor food hygiene (53.88%) and poor body hygiene (52.26%).
Recommendations
There is a need for community mobilization towards provision and use of safe and adequate water supply, latrine construction to reduce open field defecation. The high overall prevalence of intestinal parasitic infections in this present study need the mass deworming in the community and establishment of good personal hygiene and environmental sanitation; public health education is also necessary on the transmission of intestinal parasitic infections in communities, participatory approaches and combined efforts from the community and health sectors are needed to control the study areas.
References
- Abad P, Gouzy J, Aury JM, Castagnone Sereno P, Danchin EG, et al. (2008) Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognit. Nature Biotechnology 26(8): 909-915.
- Abah AE, Arene FOI, Okiwelu SN (2015) Assessment, mapping and prediction of the spatial distribution of intestinal parasitic infections in Rivers State, Nigeria. Journal of Tropical Diseases & Public Health 2(2): 1-7.
- Abate A, Kibret B, Bekalu E, Abera S, Teklu T, et al. (2013) Cross-sectional study on the prevalence of intestinal parasites and associated risk factors in Teda health centre, northwest Ethiopia. ISRN parasitology 2013(4): 10-15.
- Abdel Rahem MA, Ahmed YA (2007) Prevalence, risk factors and intestinal parasitic infections among rural school children in Sohag governorate. Ethiopian Journal Hospital Medecin 29(3): 616-630.
- Abdullah I, Hidayatullah T, Fayaz A, Nazima G, Shafaquat N, et al. (2016) Predominance of gastrointestinal protozoan parasites in children: A brief review. Journal of Health Education Research & Development 4(194): 2380-5439.
- Acevedo N, Erler A, Briza P, Puccio F, Ferreira F, et al. (2011) Allergenicity of Ascaris lumbricoides tropomyosin and IgE sensitization among asthmatic patients in a tropical environment. International Archives of Allergy and Immunology 154(3): 195-206.
- Adam RD (2001) Biology of giardia lamblia. Clinical Microbiology Reviews 14(3): 447-475.
- Alamir M, Awoke W, Feleke A (2013) Intestinal parasites infection and associated factors among school children in Dagi primary school, Amhara National Regional State, Ethiopia. Journal of Tropical Medicin 5(10): 1697-1701.
- Al Anazi AD, Alyousif MS (2011) Prevalence of gastrointestinal parasites of in Riyadh region of Saudi Arabia. Saudi Journal of Biological Sciences 18(3): 299-303.
- Albonico M, Allen H, Chitsulo L, Engels D, Gabrielli AF, Savioli L (2008) Controlling soil-transmitted helminthiasis in pre-school-age children through preventive chemotherapy. Negligated Tropical Disease 2(3): 126-132.
- Ali I, Mekete G, Wodajo N (2017) Intestinal parasitism and related risk factors among students of Asendabo elementary and junior secondary school, South Western Ethiopia. The Ethiopian Journal of Health Development (EJHD) 13(2): 7-11.
- Aka DNA, Kouadio Yapo GC, Dou SPG, Zika DK, Loukou SPK (2016) Prevalence of intestinal parasitic infections among maids in Abidjan, Côte d’ Journal of Bacteriology & Parasitology 7(2): 263.
- Akua OF, Isaac A, Michael O, Kathrine KG, Prince J Pappoe A, et al. (2017) Intestinal parasitic infections and risk factors: A cross sectional survey of some school children in a suburb in Accra, Ghana. Biomedecine Research Notes 10(1): 485.
- Andargie G, Kassu A, Moges F, Tiruneh M, Huruy K (2008) Prevalence of bacteria and intestinal parasites among food-handlers in Gondar Town, Northwest Ethiopia. Journal of Health, Population and Nutrition 26(4): 451-445.
- Anderson DJ, Reeve J, Gomez JEM, Weathers WW, Hutson S, Cunningham HV, Bird DM (1993) Sexual size dimorphism and food requirements of nestling birds. Canadian Journal of Zoology 71(12): 2541-2545.
- Anderson RM, May RM (2009) Helminth infections of humans: mathematical models, population dynamics, and control. Advances in Parasitology 24(4): 1-101.
- Ashok R, Suguneswari G, Satish K, Kesavaram V (2013) Prevalence of intestinal parasitic infection in school going children in Amalapuram, Andhra Pradesh, India. Shiraz E-Medical Journal 14(4): 1-10.
- Asrat AY, Tewodros DE, Alemayehu WO (2011) Prevalence and risk factors of intestinal parasites among delgi school children, North Gonder. Ethiopian Journal of Health and Biomedical Sciences 3(1): 75-81.
- Assafa D, Kibru E, Nagesh S, Gebreselassie S, Deribe F, et al. (2006) Medical parasitology. Ethiopia Public Health Training Initiative 135(2): 301-322.
- Ayanda OS, Ayanda OT, Adebayo FB (2010) Intestinal nematodes: A review. Group 2(1): 4-12.
- Baxt LA, Singh U (2008) New insights into Entamoeba histolytica Current Opinion in Infectious Diseases 21(5): 489-494.
- Beebe N, Marriott D, Ellis J, Harkness J (2007) Laboratory diagnostic techniques for Entamoeba Clinical Microbiology Reviews 20(3): 511-532.
- Bekalu E, Abera S, Teklu T, Yalew A, Tekeste Z (2013) Cross-sectional study on the prevalence of intestinal parasites and associated risk factors in Teda health centre. Northwest Ethiopia: SRN Parasitology 3(1): 502-506.
- Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, et al. (2006) Soil-transmitted helminth infections: Ascariasis, trichuriasis and hookworm. Shiraz E-Medical Journal 367(9521): 1521-1532.
- Bray RS, Harris WG (1977) The epidemiology of infection with Entamoeba histolytica in the Gambia, West Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene 71(5): 401-407.
- Carneiro FF, Cifuentes E, Tellez Rojo MM, Romieu I (2002) The risk of Ascaris lumbricoides infection in children as an environmental health indicator to guide preventive activities in Capara and Alto Capara, Brazil. Tropical Biomedicine 80(1): 40-46.
- Chala B (2012) A retrospective analysis of the results of a five-year (2005-2009) parasitological examination for common intestinal parasites from bale-Robe Health Center, Robe Town, Southeastern Ethiopia. ISRN Parasitology 2013.
- Chau HLQ, Thong HT, Van CN, Hung PHS, Van HV, et al. (2014) Microbial and parasitic contamination on fresh vegetables sold in traditional markets in Hue city, Vietnam. Journal of Food and Nutrition Research 2(12): 959-964.
- Cheesebrough M (2005) District laboratory practice in tropical countries. Cambridge 8(6): 654-765.
- Dawit A, Ephrem K, Nagesh S, Solomon G, Fetene D, et al. (2006) Medical parasitology. Ethiopia Public Health Training Initiative 1(1): 84-86.
- De Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D (2003) Soil-transmitted helminth infections: Updating the global picture. Trends Parasitology 19(12): 547-551.
- Dixon R, Khan SA, Gunawardena K, Kihara H, Smith JL, et al. (2015) The epidemiology of soil-transmitted helminths in Bihar State, India. Shiraz E-Medical Journal 9(5).
- Dold C (2012) Nematoda: Ascaris lumbricoides. Reports in Parasitology 79(1): 159-217.
- Emile N, Bosco NJ, Karine B (2013) Prevalence of intestinal parasitic infections and associated risk factors among Kigali institute of education students in Kigali, Rwanda. Tropical Biomedicine 30(4): 718-726.
- Eric LW (2001) Tirchuris trichiura. Medicine journal 1(2): 7-10.
- Erismann S, Diagbouga S, Odermatt P, Knoblauch AM, Gerold J, et al. (2016) Prevalence of intestinal parasitic infections and associated risk factors among school children in Burkina Faso. International Medical Journal 9(1): 554.
- Fotedar RS (2007) Laboratory diagnostic techniques for Entamoeba Clinical Microbiology Reviews 20(3): 511-532.
- Fotedar RS, Stark D, Beebe N, Marriott D, Ellis J, Harkness J (2007) Laboratory diagnostic techniques for Entamoeba Clinical Microbiology Reviews 9(1): 5-11.
- Gasser TH (2009) Improved molecular diagnostic tools for human hookworms. Expert Review Molecula Diagnosis 9(1): 17-21.
- Gebreyesus A, Adane K, Negash L, Asmelash T, Belay S, et al. (2014) Prevalence of Salmonella typhi and intestinal parasites among food handlers in Mekelle university student cafeteria, Mekelle, Ethiopia. Canadian Medicial Journal 44(6): 45-48.
- Gonçalves MLC, Araújo A, Ferreira LF (2003) Human intestinal parasites in the past: New findings and a review. England Medical Journal 98(1): 103-118.
- Greer GJ, Ow Yang CK, Yong HS (1988) Schistosoma malayensis. sp. a Schistosoma japonicum-complex Schistosoma from peninsular Malaysia. Journal of Parasitology 5(7): 471-480.
- Gupta YK (2004) Geohelminth infections (ascariasis, trichuriasis, and hookworm): Cognitive and developmental impacts. Pediatric Infectious Diseases 11(4): 245-251.
- Haftu D, Agedew F (2014) Prevalence and determinant factors of intestinal parasites among school children in Arba Minch town, Southern Ethiopia. American Journal of Health Research 2(5): 247-254.
- Haque R (2007) Human intestinal parasites. Journal of Health Population and Nutrition 25(4): 387-391.
- Haque R, Huston CD, Hughes M, Houpt E, Petri WA (2003) Amebiasis. New England Journal of Medicine 348(16): 1565-1573.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...