
Lupine Publishers Group
Lupine Publishers
Menu
Short Communication(ISSN: 2641-6875) 
Signaling Responses and Their Role in the Mitigation of Abiotic Stresses in Wheat Volume 3 - Issue 3
Faiçal Brini*
- Biotechnology and Plant Improvement Laboratory, University of Sfax, Tunisia
Received:March 16, 2023; Published:March 31, 2023
*Corresponding author:Faiçal Brini, Biotechnology and Plant Improvement Laboratory, Centre of Biotechnology of Sfax, University of Sfax, Tunisia
DOI: 10.32474/CTBM.2023.03.000165
Abstract
Wheat is one of the world’s most consumed cereal grains. During abiotic stresses, the physiological and biochemical alterations in the cells reduce growth and development of plants that ultimately decrease the yield of wheat. Thus, understanding the effects of these stresses becomes indispensable for wheat improvement programs which have depended mainly on the genetic variations present in the wheat genome through conventional breeding. Notably, recent biotechnological breakthroughs in the understanding of gene functions and access to whole genome sequences have opened new avenues for crop improvement. Despite the availability of such resources in wheat, progress is still limited to the understanding of the stress signaling mechanisms using model plants such as Arabidopsis, rice and Brachypodium and not directly using wheat as the model organism. This chapter presents an inclusive overview of knowledge on the identified mechanisms of perception and signal transduction in wheat. Specifically, this chapter provides an in-depth analysis of different signaling mechanisms observed during abiotic stress response in wheat.
Keywords: Abiotic stresses; abscisic acid; signaling molecules; Transcription factors; wheat
Introduction
Agriculture as an occupation depends on the ability to cultivate crops suitable for a particular climate in a defined region. Prolonged exposure to high temperatures in rainfed areas of the world may lead to drought stress. Exposure to high temperatures may also induce osmotic stress if water evaporates from soils resulting in elevated salt concentrations. Although drought and salt stress are the major stressors affecting crop production worldwide, the presence of a combination of these as well as heat is not uncommon and could lead to a drastic reduction in crop fitness and productivity. Among the main staple crops across the globe, cereals such as wheat, rice and maize are the most important for providing daily calories and protein intake. Of these, wheat was the first crop to be domesticated and forms the major staple food globally [1]. Wheat is the vital staple that provides around 20% of the calories and >25% of the protein to humans [2]. It occupies 30% of world cereal production (734 million tons) from 214 million hectares [3]. It provides quite a satisfactory level of dietary fiber [4] and is con sidered a nutritious food grain [5,6]. Future predictions suggested that there will be an increased demand for wheat by about 60% in 2050 to feed an estimated 9.7 billion population in the world [7].
However, during the last decade, wheat productivity enhancement was not satisfactory to meet the future demand as it was increased by only 1.1% [8]. Recently, several international organizations such as IPCC, International Maize and Wheat Improvement Centre (CIMMYT)-International Centre for Agricultural Research in Dry Areas (ICARDA), and The Organization for Economic Co-operation and Development (OECD)-Food and Agriculture Organization (FAO) have forecasted that the extreme events of abiotic stresses such as drought, high temperature, salinity, etc. will reduce wheat yield by 20–30% especially in the developing countries. Additionally, global climate change has caused various biotic (such as wheat blast disease [9,10] and hostile environmental events such as drought, thermo-stress, erratic rain, hailstorm, and salinity which instigated yield loss. Conventional breeding to improve stress tol erance is time and labor intensive and involves multigene families that govern the molecular and physiological mechanisms. Interestingly, this mechanistic complexity is further magnified due to the striking differences in the tolerance levels of different cultivars under similar stress conditions.
One of the key principles of crop improvement involves utilizing the genetic variation in the gene pool and identifying the desired traits important for attaining stress tolerance. Bread wheat (Triticum aestivum) is a hexaploid, that is composed of three closely related, but independently maintained genomes: Triticum urartu (the A-genome donor), Aegilops speltoides (the B-genome donor) and Aegilops tauschii (the D-genome donor), formed as a result of a series of naturally occurring hybridization events. The natural genetic variation in the germplasm has helped breeders to introgress new traits but has attained limited success due to the redundancy in the genomes [11,12]. However, recent biotechnological breakthroughs in the understanding of gene functions and the access to whole genome sequences have unearthed new avenues for crop improvement. Interestingly, despite the availability of such resources in wheat, progress is still limited to the understanding of the stress signaling mechanisms using model plants such as Arabidopsis, rice (monocot) and Brachypodium (as a model plant for wheat). Considerable research and development in employing these biotechnological breakthroughs have lacked thus far in wheat improvement. Stress-tolerance response is the coordinated interaction of various molecular signaling pathways involving the perception of stress and downstream activation of regulatory proteins.
Environmental signals are first sensed by a receptor/sensor present on the membrane and transduced downstream to the nucleus by a complex signaling system triggering various genes in stress response (Figure 1). The products expressed by stress response genes eventually participate in adaptation and mitigation of plant. The outcomes of the primary signaling pathway may contribute to the generation of regulatory molecules, which can initiate another signaling pathway, although with some common components involved. Most of the stress-responsive signaling pathways are regulated by the regulatory components like transcription factors (TFs), protein kinases, membrane ion channels, protein modifiers (lipidation, methylation, ubiquitination, and glycosylation), and adaptors. Systemic studies on the mechanism of signal transduction pathway at molecular level in response to abiotic stress are of vital importance for the sustainable advancement of plant breeding approaches in agriculture. In this review, we discuss the role of various signaling pathways in the mitigation of salt, drought, cold, and waterlogging stress. Additionally, this chapter aims to summarize the role of various signaling molecules and pathways that involves various stress-responsive genes in model and crops.
Figure 1: Stress signal perception and gene expression during abiotic stresses in plants. The external stress signals are sensed by the plasma membrane receptors and trigger signaling pathways. These signaling pathways lead to cellular adjustments through the expression of genes involved in stress response and development of stress mitigators.

Signaling Response in Salt Stress
Stress caused by salt is one of the abiotic hazards for plant growth and development. In the last three decades, advances in understanding salt stress signaling systems and their regulatory pathways have been increased based on genetic and biochemical studies. Stress caused by salt leads to disruption in cellular ionic and osmotic homeostasis, and their readjustments are important for sustaining regular cellular processes. During salt stress, regulation and maintenance of cellular ion homeostasis are a vital adaptive trait of salt-tolerant plants. Cellular mechanisms to decrease cytoplasmic Na+ comprise limiting Na+ uptake, increasing Na+ efflux, and transporting Na+ from the cytoplasm into the vacuole. At present, an accepted model for salt sensing hypothesizes that increased levels of reactive oxygen species (ROS) can be sensed by a ROS receptor and further transduced to activate stress-responsive genes. Recently it is proved that perception of high salinity occurs through direct binding of monovalent Na+ to the glycosyl inositol phosphoryl ceramide (GIPC). Na+ binding to GIPCs leads to the activation of unknown Ca2+ influx channels resulting in the activation of the downstream pathways. However, the components and mechanisms connecting the sensation of extracellular Na+ and Ca2+ influx are still unknown.
Salt stress activates the evolutionarily conserved salt overly sensitive (SOS) pathway to exclude Na++ ions out of cells (Figure 2). Elevated Ca2++ spikes drive an adaptive response in which Ca2++ binds and activates both SOS3 and SOS3-like Ca2++-binding protein (SCaBP8). SOS3 activates a protein kinase enzyme SOS2, which, in turn, activates the antiporter SOS1 to exclude Na++ out of the cell [13]. SOS2 can substantially increase Na+/H+ exchanger (NHX) antiporter activity during stress conditions. Detailed analyses have further demonstrated SOS2 role in the compartmentalization of Na++ ions from the cytoplasm into vacuole through coordinated interaction with CBL10 protein (Tanpure et al., 2021). Salt redistribution being of crucial mechanism in salt detoxification, as shown by various studies on Na+ transporters such as high-affinity K transporter 1 (HKT1) and Na++/H++ exchangers (NHX). HKT1 is localized in the plasma membrane and regulate cellular Na++ homeostasis, for maintaining an optimum K+/Na++ balance in the cytoplasm in response to salt stress. HKT1 is mostly expressed in vascular tissues throughout the plant in xylem parenchyma and guards from toxic effects of Na+ ions. HKT1 increases K++ ions in shoot tissue by decreasing the Na+ level through unloading into xylem parenchyma cells.
Figure 2: Schematic representation of signaling response for mitigation of salt stress in plants. The salt overly sensitive (SOS) pathway plays essential roles in Na+ exclusion. The calcium sensor, SOS3/SCaBP8, recruits SOS2 to the plasma membrane and promotes SOS2-mediated phosphorylation of the Na+/H+ antiporter. GIPCs-mediated Ca2+ influx is required for the activation of the SOS signaling pathway. MAP kinase cascades, including MAPKKK, MKK2, MKK4, MPK3, MPK4, and MPK6, are involved in the relay of salt stress signals.
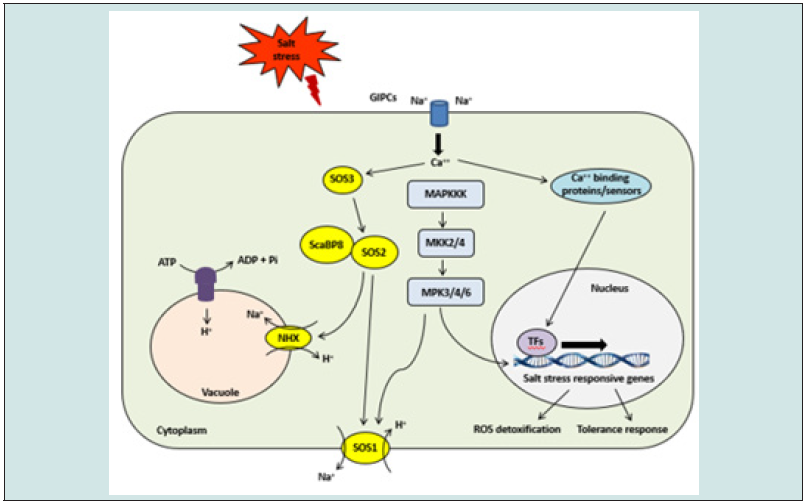
Figure 3: ABA-independent signaling pathway in response to cold and heat stress. The stress is perceived by receptor/ion channel such as OSCA1 and COLD1 present on the plasma membrane. ABA-independent signaling in response to cold stress is mediated by the ICE1 signaling cascade involving DREB. Heat stress can activate stress response through induction of ROS. This leads to activation of MPKs, protein kinases (PKs) and other phosphatases, that can trigger the Heat Shock Factors (HSF), which is kept from entering the nucleus by HSPs (HSP70/90 complex). An increase in misfolded cytosolic proteins following heat shock can destabilize this complex allowing HSF to enter the nucleus and induce transcription of stress responsive genes by binding to their HSE.

Molecular studies have identified class I and II group HKT transporters, which mediate cellular transport of Na+- and Na+-K+ cotransport, respectively. HKT1 (TaHKT2;1) gene was first isolated from wheat (Triticum aestivum), which regulates Na+/K+ transport and has led to the identification of several HKT transporters from various plant species. For example, HKT type transporters in Arabidopsis AtHKT1 and rice OsHKT1;5 mediates removal Na+ from the xylem during high salinity. In Arabidopsis, HKT1 also participates in long distance transport of Na+ through vascular tissues. SOS pathway regulates Na+/K+ homeostasis. SCaBP8 is an inhibitor of the K+ channel (AKT1) activity and through interaction with the C-terminus of AKT1. Similarly, ABA insensitive 4 (ABI4) TF is a negative regulator of AtHKT1 expression in root. HKT1 has also been shown to interact with the SOS pathway to regulate Na+/K+ homeostasis in plant cells. The Na+-selective transporters in tomato, that is, HKT1; 2 showed to mitigate salt stress through suppressing Na+ aggregation and increasing K+ homeostasis in shoots [14]. Positively charged Na+ of excess salt binds to negatively charged glycosyl inositol phosphoryl-ceramides (GIPCs) situated on plasma membrane. Na+-bound GIPCs activate Ca2+ channel that subsequently results in Ca2+ influx.
Higher Ca2+ levels are recognized by SCaBP8 and SOS3, which, in turn, activates the SOS2 protein. Activated SOS2 activates SOS1 to extrude Na+ out of the cell to regulate ionic homeostasis. H+-ATPase and NHX activities are also stimulated by SOS2 protein. Ca2+-dependent and MAPK signaling pathways also participate during the salt-stress response. Vacuolar Na+ compartmentalization is a key cellular mechanism for decreasing cytoplasmic ion toxicity in plants grown under salt stress. This adaptive mechanism is conserved in halophytic and glycophytic plants. NHX proteins localized in the tonoplast are important for the reduction of Na+ toxicity via sequestration of Na+ within the vacuole. Vacuolar transportation of Na+ is facilitated by the NHX present in vacuoles, through proton gradient formed by vacuolar H+-ATPase and H+-pyrophosphatase. AtNHX1 was the first identified plant transporter that mediates vacuolar Na+ influx. Tomato NHX3 and NHX4 are identified in the establishment of cellular ion homeostasis [15]. NHX antiporters participate in long-distance transport of Na+ from root to shoot during salt stress. Furthermore, several studies have demonstrated the crucial role of NHX antiporters in osmotic homeostasis, growth, and development.
NHX transporters are important for cellular processes such as vesicular trafficking and protein modifications. NHX1 and NHX2 located in tonoplast play a key role in the active K+ influx for turgor maintenance. Vacuolar H+-ATPase and NHX activity are positively regulated by SOS2 and elevate their exchange activity during salt stress. Stimulation of stress-induced osmotic signaling pathways induces the biosynthesis of nontoxic, highly soluble, low molecular weight compounds called osmolytes. Osmolytes maintain cellular osmotic potential, protein stability, and membrane integrity. The increased level of osmolytes such as proline, glycine, betaine amino acid, and carbohydrates enhances tolerance to osmotic stress. Osmotic stress pathways are common to most stress conditions and are not specifically stimulated by high salinity. Ca2+-permeable channel such as reduced hyperosmolality induced calcium ion increase 1 (OSCA1), regulated by osmotic changes, is a sensor during osmotic stress. The K+ exchange antiporter (KEA)1/2 and KEA3 transporters localized in plastids mediates accelerated Ca2+ responses due to osmotic stress. SNF1-related kinases (SnRK) isoforms are known to be involved in mediating osmotic stress conditions. Salt-induced stresses also include the production of ROS.
ROS are comprised of free radicals such as superoxide, hydroxyl, and nonradicals comprising singlet oxygen and hydrogen peroxide (H2O2). Plants mitigate the toxicity created from ROS by employing the antioxidant system. Detoxification signaling pathway plays a critical part in ROS scavenging and repair. ROS has also been shown to regulate Ca2+ channels. Osmotic stress induces enzymatic and nonenzymatic systems to mitigate ROS stress. Enzymatic scavengers consist of glutathione reductase (GR), glutathione-S-transferase (GST), superoxide dismutase (SOD), and ascorbate peroxidase (APX), whereas nonenzymatic scavengers involve ascorbic acid, phenolic compounds, and secondary metabolites. Protein kinases participates in response to abiotic stress by perceiving signals, triggering signal transduction pathways through the activation of downstream genes. The mitogen-activated protein kinase (MAPK) family is a protein kinase group that is well conserved in plants. Plant MAPKs are involved in signaling pathways in counter to abiotic and biotic stresses. MAPKs direct reprogramming of various cellular processes in the nucleus and mitochondria, which ultimately results in various physiological and biochemical alterations. MAPKs can be activated by stress signals such as ROS, abscisic acid (ABA), and osmotic stress.
A typical MAPK cascade is composed of three sequentially phosphorylating and activating protein kinases, a MAP kinase kinase (MAPKKK/MEKK, or MAP3K), a MAP kinase kinase (MAPKK/ MKK, or MAP2K), and a MAP kinase (MAPK). Sequence homology studies have revealed around 60 members of MAPKKKs, 10 members of MAPKKs, and 20 members MAPKs in Arabidopsis. About 15 MAPKs have been found in rice, and 19 in maize. In Arabidopsis, the MAPK cascade involving MKK2, MPK4, MPK6, and MEKK1 regulates signaling responses during salt stress. During abiotic-stress counter-action process, MAPKKKs protein activates MAPKKs by phosphorylation at serine/threonine residues, which, in turn, activates a MAPK through phosphorylation at threonine and tyrosine residues in conserved motif of MAPKs. MAPK proteins, along with phosphatases, regulate the function of various downstream proteins such as TFs and cytoskeletal proteins. During the salt stress response, phosphatidic acid has been shown to activate SOS1 antiporter activity via interaction with MPK6 in Arabidopsis. SnRK2, in reaction to osmotic stress, also activates MAPK1 and MAPK2. MAPK cascades in tomato have shown a key role in signaling response toward salt stress [16].
In abiotic-stress conditions, Ca2+ acts as a secondary messenger in the signal transduction pathway that is identified by Ca2+-binding proteins or Ca2+ sensors mediating downstream activation of cellular adaptation. Moreover, these activated Ca2+ binding proteins interact with other regulatory proteins and activate downstream signaling comprising gene expression, ion transport alteration, and posttranslational modification. Several Ca2+ binding proteins, namely, calmodulins (CaMs), CaM-like proteins (CMLs), calcineurin B-like proteins (CBLs), and Ca2+-dependent protein kinases (CDPKs) are functionally characterized. CDPKs are multigene family members that are known to be triggered in response to osmotic stress and act as crucial sensors of cellular Ca2+. CDPKs are serine/threonine protein kinases consisting of CaM-like domain- containing EF-hand domain at C-terminal, a middle kinase domain, and N-terminal domain. Upon stress sensing, elevated Ca2+ interacts with EF-hand leading to conformational changes resulting in CDPK activation. CBL-interacting protein kinase (CIPK) is another member of Ca2+-binding protein kinases participating in Ca2+-dependent salt stress signaling.
In Arabidopsis, 10 CBLs and 26 CIPKs genes showing diverse CBLCIPK interactions are identified. CIPKs perform transduction of Ca2+ signals through phosphorylation of target proteins such as the ion transporters. Another study demonstrated that high salinity triggers phosphorylation of the K+ channel TPK1 located in vacuoles through CDPK and modifies K+ transport rate into the cytosol. In rice, 10 CBLs and 33 CIPKs are identified. Further studies showed that transgenic rice overexpressing OsCIPKs exhibited enhanced tolerance against multitude of stresses like drought, salt, and cold stress suggesting their multiple functional roles in stress tolerance. CBL proteins are known to coordinate with CIPKs to regulate their activity. CBLCIPK system is involved in different signal transduction pathways, for example, salinity, drought, and cold responses. CBL1/9-CIPK23 also regulates the AKT1 potassium channels activity. Dubrovina et al. [17] demonstrated that VaCPK21 acts as a positive regulator in tolerance response to salt stress in grapes by the induction of salt-induced genes. In tomato, various CBLs have been induced during salt, drought, and cold stress [18]. Expression pattern analysis in Chinese cabbage showed CDPK-SnRK genes participate in signaling response during cold, salt, high temperature, and osmotic stress [19]. CaM and CML genes have shown deferentially expressed genes under cold, drought, and salt stress in wild tomato [20].
Signaling Response in Drought Stress
Drought induces intricate responses in plants, comprising closure of stomata, decreased turgor pressure, and lowered photosyn thesis rates, resulting in decreased growth and development. Due to the response complexity of plant to water-deficit conditions, true sensors of drought cannot be identified, even though numerous sensing mechanisms have been suggested. Drought is likely sensed by several transmembrane proteins such as OSCA1 and CSC1, which are triggered by increased plasma membrane tension leading to the influx of Ca2+ [21]. However, the exact functional role of CSC1/OSCA1 in plants needs further investigation. Transcriptional regulatory pathways involved in stress response to drought are the ABA-independent pathway regulated by DREB TFs and the ABA-dependent pathway, mediated by AREB/ABF, NAC, and MYB TFs families. ABA-responsive element binding proteins/ factors (AREB/ABF) are plant TFs belonging to the family of basic leucine zipper (bZIP) and function in ABA-dependent signaling under drought stress. AREB/ABF regulates drought tolerance at the cellular level by inducing expression of stress-related genes. In Arabidopsis, 78 bZIP TFs have been identified and classified into 10 - 13 groups. ABA-inducible genes are activated by the cis-element sequence called ABA-responsive element (ABRE) in their promoter region.
In response to ABA, AREB/ABF binds to the ABRE, which hereby trigger ABA-dependent gene expression. In AREB/ABF regulated signaling network, an elevated cellular ABA level in response to drought is sensed by the ABA receptor complex, that is, PYR/PYL/ RCARs (pyrabactin resistance/pyrabactin resistance 1-like/regulatory component of ABA receptors) and leads to the inhibition of protein phosphatase 2C (PP2C) activity. PP2C acts in regulating ABA signaling negatively and dephosphorylates SnRK2s. Activated SnRK2 phosphorylates and activates AREB/ABF TFs, which bind to the cis-acting element of ABRE in the promoters and invoke expression of stress-associated genes. SnRK2s have shown to phosphorylate and colocalize with AREB/ABFs in the nucleus. Universal stress protein (USP) isolated from wild tomato has been shown to increase drought tolerance response by significantly increasing the ABA level in transgenic tomato [22]. Dehydration-responsive element-binding proteins (DREBs) are vital plants TFs, controlling expression of several stress-inducible genes, thus representing a major ABA-independent pathway in drought stress.
It has a very significant role in the creation of tolerance against stress in plants through interacting with cis-element called DRE/ CRT sequence in the segment of promoter of stress-inducible genes. DRE-binding protein 1/CRT-binding factor (DREB1/CBF) and DREB2 are key DREB subfamily that recognizes DRE/CRT core sequence and regulates downstream gene expression. DREB1 and DREB2 participate in two independent signaling pathways consisting of cold and drought responses, respectively. Both DREB1/CBF and DREB2 are members of the APETALA2/ethylene-responsive element-binding factor (AP2/ERF) type TFs and forms a plant-specific conserved subgroup. In Arabidopsis, DREB2A and DREB2B are significantly expressed against osmotic stress caused by various abiotic conditions. DREB1/CBF protein activities are mediated through transcriptional mechanisms, whereas further regulation via proteolysis by the 26S proteasome is required in activating the DREB2A-mediated gene transcription. Apart from functional genes such as those encoding antioxidant proteins or late embryogenesis abundant (LEA) enzymes, DREB2A also regulates regulatory genes, such as TFs. Arabidopsis and transgenic rice plants overexpressing rice DREB1A showed greater tolerance to cold, salinity, and drought conditions suggesting that DREBs/CBFs are involved in the gene-regulation in multiple stress [23].
AREB/ABFs are involved in the induction of DREB2A and indicate the cross talk between ABA-independent and ABA-dependent pathways. Several transgenic plants expressing DREB TFs showed significantly improved tolerance against drought, high salinity, and cold. For example, dwarf apple MbDREB1 TF has evidenced increased tolerance to salt, drought, cold stress through activation of ABA-dependent and ABA-independent genes in transgenic Arabidopsis [24]. Gene expression analysis of drought-stressed peach leaves showed upregulation of DREB and WRKY genes [25]. NAC is another plant-specific, one of the largest TFs gene family, involved in plant developmental processes and stress response. NAC TFs are induced during high salt, cold, ABA, ethylene, and methyl jasmonate, suggesting considerable cross talk between various stresses. NAC proteins contain highly conserved NAC domains and variable domains at C-terminal, which are responsible for DNA-binding activity and transcription activity, respectively. The DNA-binding domain of the NAC TF targets the NAC recognition sequence, containing cis-acting elements in the promoter region of several stress-induced genes. NAC TFs activate the transcription of the early responsive to dehydration stress 1 (ERD1) gene via the ABA-independent pathway. Also, NAC TFs are induced by ABA and upregulate the ABA-inducible genes. Rice SNAC genes, that is, OsNAC5, OsNAC6, and SNAC1 are involved in salt, drought, and cold stress and have been shown to be inducible by ABA.
The aforementioned evidences suggest a crucial role played by NAC TFs in stress tolerance and the successful survival of plants in both ABA-independent and ABA-dependent pathways. Tomato WRKY81 has been shown to negatively regulate the drought-tolerance response through the aggregation of H2O2 in the guard cells [26]. Overexpression of Banana NAC68 TF in transgenic banana lines has been shown to enhance drought as well as salt tolerance through altered expression of auxin-responsive factors and IAA-inducible genes [27]. Several genetic and biochemical studies have reported the key role of Ca2+ in drought stress. Ca2+ is one of the crucial second messengers involved in drought stress signaling via ABA-dependent and ABA-independent pathways by activating different protein kinases such as the CDPK family. CDPKs are shown to have a critical role in stomatal closure and induction of gene expression in ABA-dependent stress signaling during drought stress. Under drought stress, extracellular Ca2+ and apoplastic ABA activates guard cell plasma membrane ion channels, which results in an intracellular Ca2+ spike. CDPKs, that is, AtCPK6 and AtCPK23, also phosphorylate SLAC1, a slow anion channel, which plays a role in stomatal closure by ion transport regulation in guard cells. CDPKs also mitigate drought stress through the inhibition of KAT1 channels. Additionally, H2O2 and NO increase cytoplasmic Ca2+ and stimulate SLAC1 channels, resulting in stomatal closure.
ABA receptor proteins ABI1 shows negative regulation of the ABA response and is repressed upon interaction with ABA. The silencing of Arabidopsis ABI1 receptors resulted in increased ABA sensitivity and decreased water intake. CDPKs shows interaction with group A protein phosphatases type 2C (PP2C), as well as SnRK2s, suggesting that alternative functional role of CDPKs in the ABA signaling pathway. In Arabidopsis, CPK3 and CPK6 genes regulate ABA functions in guard stomatal cells whereas CPK4 and CPK11 show positive regulation of ABA responses. Additionally, CPKs phosphorylate AREB/ABF TFs in the ABA-dependent pathway. Overexpression of grape VaCIPK02 protein in Arabidopsis displayed enhanced tolerance to drought stress through increased accumulation of ABA and decreasing cellular ROS levels [28]. MAPK signaling cascade is another player responding to drought stress. Transcriptional studies of MAPK cascade showed that Arabidopsis MPK2, MPK3, MPK4, MPK5, MPK12, and MAPKKK4 are invoked in drought conditions [29]. In Gossypium hirsutum, MAPK genes have shown their potential in enhancing drought and salt stress tolerance [30]. Similarly, MAPK signaling genes have been shown to participate during drought stress in grapevine and pepper. GWAS and transcriptome studies identified 21 MAPK genes from cassava during drought stress [31]. Transcriptome studies in cassava revealed significant differential expression of MAPKKK genes suggesting its role in drought tolerances [32].
Signaling Response in Cold Stress
Plants perceive cold signals and activate downstream signal transduction pathways to induce stress-responsive genes. The potential cold signal sensors in plants comprise Ca2+ regulated channels, histidine kinases, and receptors associated with G-proteins. Furthermore, an alternation in temperature leads to a modification in membrane fluidity and cytoskeleton organization, subsequently followed by Ca2+ influx, which leads to downstream signaling. Downstream responses consist of the general transcriptional regulation pathways such as Ca2+ signaling pathways, MAPK cascades, and C-repeat binding factor (CBF)-dependent or CBF-independent pathways. Ca2+ signaling relays cold-signaling responses through different Ca2+-binding proteins. CaMs, CBLs, and CDPKs play important roles as Ca2+ sensors in response to cold stress. CaMs consist of a small, conserved gene family and well characterized small acidic Ca2+-binding protein. CaMs have four EF-hand domains and the binding of Ca2+ causes change in conformation that leads in the activation of CaMs interacting proteins. Additionally, MAPK cascade is also governed by CaMs. Similarly, Calcineurin B-like (CBL) proteins also known as SOS3-like Ca2+-binding proteins (ScaBLs), also act as Ca2+ sensors. During cold conditions, CBL proteins act as plants Ca2+ sensors that regulate cellular Ca2+ levels by interacting with CIPKs. For instance, evidence suggests that Arabidopsis CBL1 plays an important role in mediating cold tolerance via interacting with cold-induced CIPK7. CIPKs are known to act downstream of Ca2+ signal but upstream of TFs for regulation of cold stress. CDPKs act as stress sensors and are positive regulators of Ca2+-mediated signaling response during cold tolerance.
MAPK cascade is crucial for signal transduction of cold-stress signals and performs distinct roles during cold-stress response in various species. The functional roles of signaling components of MAPKs cascade during cold stress have been mostly investigated in Arabidopsis. Ca2+/calmodulin-regulated receptor-like kinases (CRLK1/2) are involved in positive regulation of cold tolerance via inhibition of (MPK3/6) activity. CRLK1 regulates cold-stress responses by phosphorylating MEKK1, which, in turn, phosphorylates MKK2 during cold treatment. MKK2 regulates the expression of cold-responsive genes to mitigate damage due to cold stress. MKK2 has also been shown to regulate MPK4 and MPK6 activity. MPK3/6 acts as negative regulators of the CBF signaling pathway via phosphorylation of ICE1 (inducer of CBF expression 1) to decrease its stability and binding activity, resulting in reduced cold tolerance. Under cold stress, ROS is known to activate signaling cascade, including ANP1, MPK3, MPK4/6, and nucleoside diphosphate kinase 2 [33]. In rice, MPK3 phosphorylates ICE1 leading to its interruption in interaction with HOS1. This results in increased stability of ICE1 protein and its ability to interact with trehalose-6-phosphate phosphatase1 (TPP1) enzyme, which catalysis the production of carbohydrate trehalose, thus increasing cold tolerance.
The ABA-independent signaling pathway responds to cold stress without the requirement of ABA activation. In Arabidopsis, cold stress has been shown to induce expression of ICE1, encoding a bHLH TF that is capable of activating downstream DREB1/CBF members [34]. In wheat, homologs of ICE1 have been identified as TaICE141 and TaICE187 which regulate CBF genes, demonstrating conservation of cold stress signaling between species [35]. Overexpression lines of ICE1 and TaICE141/TaICE187 in Arabidopsis subjected to cold stress demonstrated the importance of ICE homologs for enhanced cold tolerance [35]. Induced DREB1A/CBF3 can then interact with DRE/CRT promoter elements to regulate the expression of various cold-responsive genes [36,37]. Persistent ICE1 activity is known to be regulated by protein modifications; simulation of ICE1 by SIZ1 can increase the stability of ICE1, driving a prolonged cold response [38]. The activation of various cold-responsive genes downstream can then mitigate the effects of cold stress. To moderate or repress the cold response, ICE1 can be polyubiquitinated by HOS1, which targets ICE1 for 26S proteasomal-mediated degradation (Figure 3) [39]. Increasing ABA content in response to cold stress can facilitate the accumulation of various second messenger molecules including Ca2+ and ROS [40]. Arabidopsis FRY1, required for inositol triphosphate (IP3) turnover, functions as a negative regulator of ABA and abiotic stress responses and functions in attenuating these responses [41].
In wheat seedlings, ABA application induces a marked increase in Ca2++, which can subsequently increase the activity of NADPH oxidase leading to accumulation of hydrogen peroxide [40]. An increase in ABA and cytosolic Ca2+ can also signal the activation of calcium-dependent protein kinases (CDPKs) to mitigate the effects of various abiotic stresses. Li et al. [42] analyzed 20 CDPKs in wheat and identified seven different CDPKs that responded to exogenous ABA treatment. From the ABA-responsive CDPKs, CDPK9 was re sponsive to drought and salinity suggesting that CDPK may play a role in abiotic response and integration of various stresses. In rice, CPK24 has involved stress response during cold tolerance, via increased accumulation of proline and phosphorylation of glutathione- dependent thioltransferase enzyme. Apart from CBF regulatory pathway, there is distinct CBF-independent signaling pathway that mediates cold responsive gene expression. Genomic analysis indicated that CBFs regulate only a limited set of cold-responsive genes, indicating that other transcriptional networks might contribute to cold-signaling response. For example, Arabidopsis eskimo1 (ESK1) mutant shows freezing tolerance through higher accumulation of proline. ESK1 has a distinct molecular basis and provides cold tolerance by regulating genes that are independent of CBF regulatory pathway [43].
Signaling Response in Heat Stress
At the cellular level, high temperature can lead to misfolding, denaturation or loss of function of proteins which affects optimal cellular functions triggering stress responses. Temperature fluctuation- induced changes in plasma membrane fluidity have been associated with the generation of PA and phosphatidylinositol 4,5-bisphosphate (PIP2). Whether PA, PIP2 or related molecules have any role in perception is unclear, but it was shown that PIP2 is generated through activation of PIPK, and PIP2 requires small monomeric G-proteins or the α-subunit of the heterotrimeric G-protein for its activation [44]. Such receptors which respond to elevated temperatures are yet to be characterized, and the role of self-activating heterotrimeric G-protein is still under investigation [45]. Heat stress may be perceived via chaperone-mediated signaling proteins, wherein, under non-stressed conditions, chaperone proteins may be bound to heat-stress response TFs (Heat shock factors; HSFs) keeping them inactive. Upon elevation of temperatures, the chaperone proteins dissociate from the heat-stress response transcription factors, and this allows them to bind to heat-stress responsive genes known as heat shock elements (HSE) and activate downstream physiological responses [46]. Under non-stressed conditions, bZIP28 (ER membrane bound TF) is bound by the chaperone BIP preventing its movement into the nucleus.
Stress-induced protein unfolding or misfolding abolishes the BIP-bZIP28 interaction that mobilizes bZIP28 to the Golgi where it is proteolytically processed for its migration to the nucleus which activates transcriptional changes [47,48]. Heat stress changes membrane fluidity which leads to rapid generation of ROS and increased Ca2+ influx [49-51]. Like other abiotic stresses, ROS and Ca2+ can trigger heat shock responses by activating the heat shock TF, HsfA1, which is referred to as the master-regulator of heat responses in plants [52]. However, the cytoplasmic pool contains multiple forms of heat shock proteins (HSPs), the levels of which are significantly upregulated under heat stress conditions [53]. The upregulation of these HSPs is controlled through the activation of HSFA1, the activation of which is proposed to occur in two possible pathways, the chaperone titration model [54] and HsfA1-independent heat shock response [55,56]. In chaperone titration model, HSP70 and HSP90 are shown to bind HsfA1, which prevents activation of HsfA1 by another regulatory protein involved in post-translational modifications of HsfA1 [57]. During heat stress, the stoichiometric increase in the abundance of unfolded proteins leads to the destabilization of the HSFA1-HSP70-HSP90 complex, ultimately releasing the HsfA1 for its entry into nucleus and inducing the transcription of heat shock response genes (Figure 4).
Figure 4: ABA-dependent signaling pathway in response to temperature stress. Under heat stress, ABA-dependent signaling is mediated by NAC, AP2/ERF, and WRKY TFs. Under cold stress, ABA can promote expression of CBF4 which can mediate transcription of downstream stress responsive genes. ABA interaction with receptor components can activate phosphorylation of ABREs which can mediate downstream gene expression.
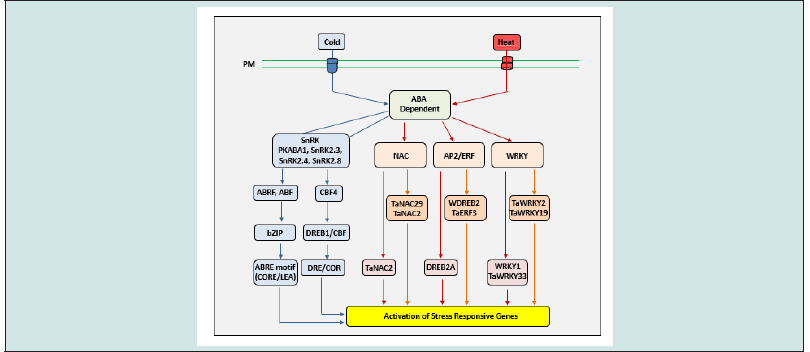
The HsfA1-independent activation occurs through an increase in response to oxidative load and free cytosolic Ca2+, where the increase in free Ca2+ levels is correlated with IP3 levels leading to activation of DREB2A [58]. The activation of DREB2A has also been observed during osmotic stress signaling, indicating that activation of HSFs is not limited to the regulation of HSPs, but otherwise forms an elaborate network that regulates a multitude of functions [59]. Compared to non-plant species such as yeast and humans which only have 1 and 3 HSFs respectively, Arabidopsis, rice, and wheat have 21, 23, and 56 HSFs respectively [60,61]. The increased numbers of these HSFs indicate that these genes are important for diverse cellular functions and likely became increasingly redundant to overlap or complement metabolic needs [62]. Interestingly, out of 56 known HSFs in wheat, only a few have been characterized for their roles in signaling. In another finding, overexpression of TaHsfC2a- B was shown to induce expression of TaHSP70d and TaGalSyn conferring tolerance to heat stress in an ABA-dependent manner [63], and TaHSP26 overexpression was shown to confer tolerance of Arabidopsis to continuous exposure to high heat [64]. Other TFs such as TaWRKY1, TaWRKY33, and TaNAC2L offered significant tolerance to heat stress when overexpressed in Arabidopsis (Figure 4) [65,66].
Modulation of Signaling Responses by Genetic Engineering Approach to Mitigate Abiotic Stresses
Genetic engineering strategies are efficiently used in crop improvement, specially to answer various stresses. It is a rapid solution compared to the traditional breeding approaches and is in practice for crop improvement. Various gene delivery methods such as Agrobacterium-mediated, as well as particle bombardment techniques, are generally used for engineering desired plant cells for the improvement of targeted traits. A huge number of literatures are obtainable on the improvement of various environmental stress tolerance in plants by genetic engineering. For example, scientists in Egypt have successfully developed drought-tolerant wheat cultivar through the transformation of ‘HVAI1’ gene from barley to wheat [67]. More work is going on to develop genetically modified drought-tolerant wheat cultivar in China and other countries [68]. Genes from wheat TNHX1 and TVP1 transferred in Arabidopsis and the transgenic exhibited enhanced tolerance to both drought and salt stresses [69].
The transgene(s) encoding key signaling factors such as TFs, protein kinases, receptor kinases, ion transporter, phytohormones, osmolytes, and detoxification enzymes involved in signaling responses have been proved to be crucial for augmenting tolerance level in stress conditions in crop plants (for review, see [70-72]). Genetic engineering of key-signaling pathways has contributed significantly for the development of transgenic stress-tolerant plants over the past decade. The conventional transgenic events are usually performed by a single-gene approach under control of the constitutive promoter. Transcription factors are the major transgenes that regulate the expression of other genes under stress. TFs can control the regulation of stress-related genes during signaling responses and, thus, have enormous potential applications in engineering stress tolerant crops. Thus, such approaches provide a new direction for the advancement of stress-tolerant transgenic plants against abiotic stresses.
In transgenic wheat, drought tolerance is improved by utilizing stress-inducible promoter rd29A with DREB1 from Arabidopsis [73]. Although hundreds of thousands of candidate genes were identified for abiotic stress tolerance, their practical utilization in developing commercial variety is very slow. The application of the revolutionary CRISPR-Cas genome editing technology should accelerate the development of new wheat varieties tolerant to multiple abiotic stresses [74]. Bhowmik et al. [75] have developed a convenient method for mutagenesis of wheat using CRISPR-Cas technology. Recent genome editing tools comprising clustered regularly interspaced short palindromic repeats/CRISPR-associated 9 (CRISPR/Cas9), zinc-finger nucleases (ZFNs), and transcription activator- like effector nucleases (TALENs) have opened many exciting opportunities to enhance stress tolerance in plants. These genetic tools have been used not only for genomic enhancement of desirable traits but also for functional validation of plant genes. Selection of inducible promoters or cell type-specific promoters instead of constitutive promoters may offer optimum levels of transgene expression and avoid the growth penalty under stress conditions.
Conclusion and Future Prospects
Global food security is a major concern being addressed worldwide. Rapid population increases and unpredictable climatic events continue to pressure the need to increase crop productivity. Climatic events can induce abiotic stresses not limited to drought and temperature stress, while diminishing soil conditions can enhance saline stress. Wheat is an important crop accounting for a large portion of human caloric consumption, therefore minimizing abiotic stress is critical to preserve global food security. Understanding perception and signaling cascades activated in response to abiotic stress will provide valuable information which can be used to design new technologies to mitigate yield loss induced by abiotic stress. Identifying novel players involved in abiotic stress signaling will allow researchers to design new technologies and farming strategies for mitigating abiotic stress. The key would be to generate new wheat germplasms that can maintain their growth vigor and yield traits while maintaining an enhanced ability to withstand adverse environmental assaults. There also appears to be potential for generating transgenic wheat varieties that have altered expression of upstream regulators or downstream stress responsive genes involved in various abiotic stresses. This, however, seems like an unlikely avenue due to the regulatory agencies and public fear of genetically modified organisms. Ultimately, the advancement and identification of novel stress signaling players will be critical for designing technologies to increase plant productivity and yield when exposed to unfavorable growth conditions.
References
- Tack J, Barkley A, Nalley LL (2015) Effect of warming temperatures on US wheat yields. Proc Natl Acad Sci USA 112: 6931-6936.
- FAO (Food and Agricultural Organization) (2018) FAO Statistical Pocket Book; Food and Agricultural Organization, Rome, Italy pp. 28.
- FAOSTAT (2020) Wheat Production Statistics.
- Giraldo P, Benavente E, Manzano-Agugliaro F, Gimenez E (2019) Worldwide Research Trends on Wheat and Barley: A Bibliometric Comparative Analysis. Agronomy 9(7): 352.
- Sarwar MH, Sarwar MF, Sarwar M, Qadri NA, Moghal S (2013) The importance of cereals (Poaceae: Gramineae) nutrition in human health: A review. J Cereals Oil seeds 4(3): 32-35.
- Shewry PR, Hey SJ (2015) The contribution of wheat to human diet and health. Food Energy Secur 4(3): 178-202.
- Yadav S, Modi P, Dave A, Vijapura A, Patel D, et al. (2020) Effect of Abiotic Stress on Crops. Sustainable Crop Production ed, Hasanuzzaman M, Fujita M, Teixeira Filho MCM, Nogueira TAR, Galindo FS, Intech Open, London, UK.
- Wheat Initiative (2018) In Annual Report: Coordinating Global Wheat Research; Federal Research Centre for Cultivated Plants: Berlin, Germany pp. 60.
- Islam T, Croll D, Gladieux P, Soanes DM, Persoons A, et al. (2016) Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biol 14(1): 84.
- Islam, T, Gupta DR, Hossain A, Roy KK, He X, et al. (2020) Wheat blast: A new threat to food security. Phytopathol Res 2: 28.
- Brenchley R, Spannagl M, Pfeifer M, Barker GL, D’Amore R, et al. (2012) Analysis of the bread wheat genome using whole-genome shotgun sequencing. Nature 491(7426): 705-710.
- Panchy N, Lehti-Shiu MD, Shiu SH (2016) Evolution of gene duplication in plants. Plant Physiol 171(4): 2294-2316.
- Tanpure RS, Ghuge SA, Dawkar VV, Kumar A (2021) Signaling responses and their role in the mitigation of abiotic stresses. Stress tolerance in horticultural crops, pp. 327-346.
- Jaime-Perez N, Pineda B, Garcıa-Sogo B, Atares A, Athman A, et al. (2017) The sodium transporter encoded by the HKT1; 2 gene modulate sodium/potassium homeostasis in tomato shoots under salinity. Plant Cell Env 40(5): 658-671.
- Galvez FJ, Baghour M, Hao G, Cagnac O, Rodrıguez-Rosales MP, et al. (2012) Expression of LeNHX isoforms in response to salt stress in salt sensitive and salt tolerant tomato species. Plant Physiol Biochem 51: 109-115.
- Nigam M, Mishra AP, Salehi B, Kumar M, Sahrifi-Rad M, et al. (2019) Accelerated ageing induces physiological and biochemical changes in tomato seeds involving MAPK pathways. Sci Hortic 248: 20-28.
- Dubrovina AS, Kiselev KV, Khristenko VS, Aleynova OA (2016) VaCPK21, a calcium-dependent protein kinase gene of wild grapevine Vitis amurensis Rupr., is involved in grape response to salt stress. Plant Cell Tiss Org Cul 124(1): 137-150.
- Kabir MH, Wang MH (2010) Stress-induced expression profiling of a calcium sensor, calcineurin B-like protein gene (SlCBL) in tomato. J Hortic Sci Biotech 85(2): 154-160.
- Wu P, Wang W, Duan W, Li Y, Hou X (2017) Comprehensive analysis of the CDPK-SnRK superfamily genes in Chinese cabbage and its evolutionary implications in plants. Front Plant Sci 8: 162.
- Jinyan S, Xiangge D (2020) Identification, characterization and expression analysis of calmodulin and calmodulin-like proteins in Solanum pennellii. Sci Rep 10(1).
- Liu X, Wang J, Sun L (2018) Structure of the hyperosmolality-gated calcium-permeable channel OSCA1. 2. Nat Commun 9(1): 1-9.
- Loukehaich R, Wang T, Ouyang B, Ziaf K, Li H, et al. (2012) SpUSP, an annexin-interacting universal stress protein, enhances drought tolerance in tomato. J Exp Bot 63(15): 5593-5606.
- Ito Y, Katsura K, Maruyama K, Taji T, Kobayashi M, et al. (2006) Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol 47(1): 141-153.
- Yang W, Liu XD, Chi XJ, Wu CA, Li YZ, et al. (2011) Dwarf apple MbDREB1 enhances plant tolerance to low temperature, drought, and salt stress via both ABA-dependent and ABA-independent pathways. Planta 233(2): 219-229.
- Haider MS, Kurjogi MM, Khalil-ur-Rehman M, Pervez T, Songtao J, et al. (2018) Drought stress revealed physiological, biochemical, and gene-expressional variations in ‘Yoshihime’ peach (Prunus Persica L) cultivar. J Plant Interact 13 (1): 83-90.
- Ahammed GJ, Li X, Yang Y, Liu C, Zhou G, et al. (2020) Tomato WRKY81 acts as a negative regulator for drought tolerance by modulating guard cell H2O2-mediated stomatal closure. Environ Exp Bot 171: 103960.
- Negi S, Tak H, Ganapathi TR (2016) Expression analysis of MusaNAC68 transcription factor and its functional analysis by overexpression in transgenic banana plants. Plant Cell Tiss Org Cul 125(1): 1-12.
- Xu W, Shen W, Ma J, Ya R, Zheng Q, et al. (2020) Role of an Amur grape CBL-interacting protein kinase VaCIPK02 in drought tolerance by modulating ABA signaling and ROS production. Environ Exp Bot 172: 103999.
- Moustafa K, Abu Qamar S, Jarrar M, Al-Rajab AJ, Tre mouillaux-Guiller J (2014) MAPK cascades and major abiotic stresses. Plant Cell Rep 33(8): 1217-1225.
- Bello SS, Gereziher MT, Adeel A, Tajo SM, Ibrahim S, et al. (2022) Genome wide identification and characterization of MAPK genes reveals their potential in enhancing drought and salt stress tolerance in Gossypium hirsutum. J Cotton Res 5(23): 1-13.
- Fan W, Hai M, Guo Y, Ding Z, Tie W, et al. (2016) The ERF transcription factor family in cassava: genome-wide characterization and expression analyses against drought stress. Sci Rep 6: 37379.
- Ye J, Yang H, Shi H, Wei Y, Tie W, et al. (2017) The MAPKKK gene family in cassava: genome-wide identification and expression analysis against drought stress. Sci Rep 7(1): 1-12.
- Teige M, Scheikl E, Eulgem T, Doczi R, Ichimura K, et al. (2004) The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell 15(1): 141-152.
- Chinnusamy V (2003) ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev 17(8): 1043-1054.
- Badawi M, Reddy YV, Agharbaoui Z, Tominaga Y, Danyluk J, et al. (2008) Structure and functional analysis of wheat ICE (inducer of CBF expression) genes. Plant Cell Physiol 49(8): 1237-1249.
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, et al. (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10(8): 1391-1406.
- Novillo F, Alonso JM, Ecker JR, Salinas J (2004) CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis. Proc Natl Acad Sci USA 101(11): 3985-3990.
- Miura K, Jin JB, Lee J, Yoo CY, Stirm V, et al. (2007) SIZ1-Mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis. Plant Cell 19(4): 1403-1414.
- Dong CH, Agarwal M, Zhang Y, Xie Q, Zhu JK (2006) The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc Natl Acad Sci USA 103(21): 8281-8286.
- Agarwal S, Sairam RK, Srivastava GC, Tyagi A, Meena RC (2005) Role of ABA, salicylic acid, calcium and hydrogen peroxide on antioxidant enzymes induction in wheat seedlings. Plant Sci 169(3): 559-570.
- Xiong L, Lee B, Ishitani M, Lee H, Zhang C, et al. (2001) FIERY1 encoding an inositol polyphosphate 1-phosphatase is a negative regulator of abscisic acid and stress signaling in Arabidopsis. Gene Dev 15(15): 1971-1984.
- Li AL, Zhu YF, Tan XM, Wang X, Wei B, et al. (2008) Evolutionary and functional study of the CDPK gene family in wheat (Triticum aestivum L). Plant Mol Biol 66(4): 429-443.
- Xin Z, Mandaokar A, Chen J, Last RL, Browse J (2007) Arabidopsis ESK1 encodes a novel regulator of freezing tolerance. Plant J 49(5): 786-799.
- Mishkind M, Vermeer JEM, Darwish E, Munnik T (2009) Heat stress activates phospholipase D and triggers PIP2 accumulation at the plasma membrane and nucleus. Plant J 60(1): 10-21.
- Urano D, Chen JG, Botella JR, Jones AM (2013) Heterotrimeric G protein signaling in the plant kingdom. Open Biol 3(3): 120186.
- Zhu JK (2016) Abiotic stress signaling and responses in plants. Cell 167(2): 313-324.
- Gao H, Brandizzi F, Benning C, Larkin RM (2008) A membrane-tethered transcription factor defines a branch of the heat stress response in Arabidopsis thaliana. Proc Natl Acad Sci USA 105(42): 16398-16403.
- Liu JX, Howell SH (2016) Managing the protein folding demands in the endoplasmic reticulum of plants. New Phytol 211(2): 418-428.
- Los DA, Murata N (2004) Membrane fluidity and its roles in the perception of environmental signals. Biochim Biophys Acta 1666(1-2): 142-157.
- Horváth I, Glatz A, Nakamoto H, Mishkind ML, Munnik T, et al. (2012) Heat shock response in photosynthetic organisms: membrane and lipid connections. Prog Lipid Res 51(3): 208-220.
- Kurusu T, Nishikawa D, Yamazaki Y, Gotoh M, Nakano M, et al. (2012) Plasma membrane protein OsMCA1 is involved in regulation of hypoosmotic shock induced Ca2+ influx and modulates generation of reactive oxygen species in cultured rice cells. BMC Plant Biol 12: 11.
- Ohama N, Kusakabe K, Mizoi J, Zhao H, Kidokoro S, et al. (2016) The transcriptional cascade in the heat stress response of Arabidopsis is strictly regulated at the level of transcription factor expression. Plant Cell 28(1): 181-201.
- Driedonks N, Xu J, Peters JL, Park S, Rieu I (2015) Multi-Level interactions between heat shock factors, heat shock proteins, and the redox system regulate acclimation to heat. Front Plant Sci 6: 999.
- Richter K, Haslbeck M, Buchner J (2010) The heat shock response: life on the verge of death. Mol Cell 40(2): 253-266.
- Volkov RA, Panchuk II, Mullineaux PM, Schöffl F (2006) Heat stress induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol Biol 61(4-5): 733-746.
- Sangwan V, Örvar BL, Beyerly J, Hirt H, Dhindsa Rajinder S (2002) Opposite exchanges in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J 31(5): 629-638.
- Hahn A, Bublak D, Schleiff E, Scharf KD (2011) Crosstalk between Hsp90 and Hsp70 chaperones and heat stress transcription factors in tomato. Plant Cell 23(2): 741-755.
- Zheng SZ, Liu YL, Li B, Shang ZL, Zhou RG, et al. (2012) Phosphoinositide-specific phospholipase C9 is involved in the thermotolerance of Arabidopsis. Plant J 69(4): 689-700.
- Ohama N, Sato H, Shinozaki K, Yamaguchi-Shinozaki K (2017) Transcriptional regulatory network of plant heat stress response. Trends Plant Sci 22(1): 53-65.
- Scharf KD, Berberich T, Ebersberger I, Nover L (2012) The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochim Biophys Acta 1819(2): 104-119.
- Xue GP, Sadat S, Drenth J, McIntyre CL (2014) The heat shock factor family from Triticum aestivum in response to heat and other major abiotic stresses and their role in regulation of heat shock protein genes. J Exp Bot 65(2): 539-557.
- Dubcovsky J, Dvorak J (2007) Genome plasticity a key factor in the success of polyploid wheat under domestication. Sci 316(5833): 1862-1866.
- Hu XJ, Chen D, Lynne Mclntyre C, Fernanda Dreccer M, Zhang ZB, et al. (2017) Heat shock factor C2a serves as a proactive mechanism for heat protection in developing grains in wheat via an ABA-mediated regulatory pathway. Plant Cell Environ 41(1): 79-98.
- Chauhan H, Khurana N, Nijhavan A, Khurana JP, Khurana P (2012) The wheat chloroplastic small heat shock protein (sHSP26) is involved in seed maturation and germination and imparts tolerance to heat stress. Plant Cell Environ 35(11): 1912-1931.
- Guo W, Zhang J, Zhang N, Xin M, Peng H, et al. (2015) The wheat NAC transcription factor TaNAC2L is regulated at the transcriptional and post-translational levels and promotes heat stress tolerance in transgenic Arabidopsis. PLoS One 10(8): e0135667.
- He GH, Xu JY, Wang YX, Liu JM, Li PS, et al. (2016) Drought-responsive WRKY transcription factor genes TaWRKY1 and TaWRKY33 from wheat confer drought and/or heat resistance in Arabidopsis. BMC Plant Biol 16(1): 116.
- Meena HP, Bainsla NK, Yadav DK (2017) Breeding for Abiotic Stress Tolerance in Crop Plants. In Recent Advances in Plant Stress Physiology; Daya Publishing House: New Delhi, India, pp. 329-378.
- Xia L, Ma Y, He Y, Jones HD (2012) GM Wheat Development in China: Current Status and Challenges to Commercialization. J Exp Bot 63(5): 1785-1790.
- Brini F, Hanin M, Mezghani I, Berkowitz GA, Masmoudi K (2007) Overexpression of wheat Na+/H+ antiporter TNHX1 and H+ -pyro phosphatase TVP1 pyrophosphatase TVP1 improve salt and drought stress tolerance in Arabidopsis thaliana plants. J Exp Bot 58: 301-308.
- An JP, Li R, Qu FJ, You CX, Wang XF, et al. (2018) R2R3-MYB transcription factor MdMYB23 is involved in the cold tolerance and proanthocyanidin accumulation in apple. Plant J 96(3): 562-577.
- Yarra R, Kirti PB (2019) Expressing class I wheat NHX (TaNHX2) gene in eggplant (Solanum melongena L.) improves plant performance under saline condition. Funct Integr Genom 19 (4): 541-554.
- Jia D, Jiang Q, van Nocker S, Gong X, Ma F (2019) An apple (Malus domestica) NAC transcription factor enhances drought tolerance in transgenic apple plants. Plant Physiol Biochem 139: 504-512.
- Pellegrineschi A, Reynolds M, Pacheco M, Brito RM, Almeraya R, et al. (2004) Stress-induced expression in wheat of the Arabidopsis thaliana DREB1A gene delays water stress symptoms under greenhouse conditions. Genome 47(3): 493-500.
- Bhowmik P, Hassan MM, Molla K, Rahman M, Islam, MT (2019) Application of CRISPR-Cas genome editing tools for the improvement of plant abiotic stress tolerance. In Approaches for Enhancing Abiotic Stress Tolerance in Plants pp. 459-472.
- Bhowmik P, Ellison E, Polley B, Bollina V, Kulkarni M, et al. (2018) Targeted mutagenesis in wheat microspores using CRISPR/Cas9. Sci Rep 8(1): 6502.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...





