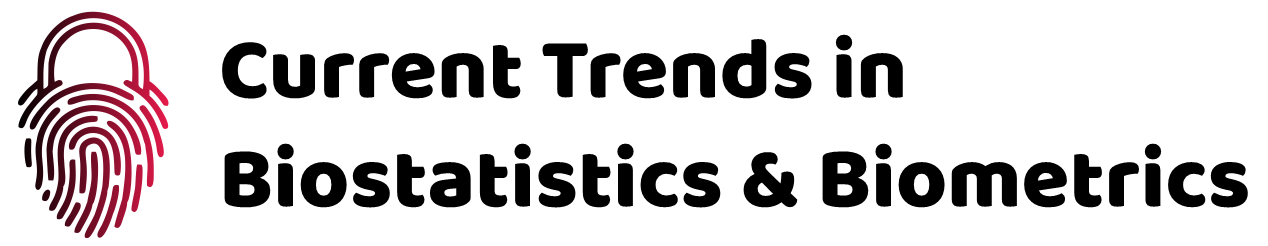Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1381
Review Article(ISSN: 2644-1381) 
The Role of Mutations on Gene RNP in Sharp Syndrome Volume 3 - Issue 4
Shahin Asadi*, Shima Dolabi, Mahtab Farrash Bashi Masjed and Sahel Kesrat
- Division of Medical Genetics and Molecular Optogenetic Research, Medical Genetics, Harvard University, USA
Received: January 31, 2022 Published: February 08, 2022
*Corresponding author: Division of Medical Genetics and Molecular Optogenetic Research, Medical Genetics, Harvard University, USA
DOI: 10.32474/CTBB.2022.03.000168
Abstract
Mixed Connective Tissue Disease (MTCD) is an uncommon systemic inflammatory rheumatic disease. MCTD is a specific subset of the broader category of rheumatic “overlapping syndromes,” a term used to describe when a patient has more than one characteristic of a classic inflammatory rheumatic disease. These classic rheumatic diseases include systemic lupus erythematosus, polymyositis, scleroderma, and rheumatoid arthritis. People with overlapping syndrome may, but do not need to meet the diagnostic criteria for one (or more) classic rheumatic diseases. MCTD is distinguished from other overlapping syndromes by a laboratory result: MCTD patients have rheumatic overlap syndrome plus anti-RNP antibodies. In addition, it has been suggested that the term “MCTD” be used to refer to patients with clinical features that include at least one of the following “common manifestations”: Raynaud’s phenomenon, swollen fingers, or swollen hands.
Keywords: Sharp syndrome; RNP Gene; Skin Disorder; Genetic Mutation
Overview of Sharp Syndrome
Sharp syndrome, also known as Mixed Connective Tissue Disease (MTCD), is an uncommon systemic inflammatory rheumatic disease. Sharp syndrome is a specific subset of the broader category of rheumatic “overlapping syndromes,” a term used to describe when a patient has more than one characteristic of a classic rheumatic inflammatory disease (Figure 1). These classic rheumatic diseases include systemic lupus erythematosus, polymyositis, scleroderma, and rheumatoid arthritis. People with overlap syndrome may have the full diagnostic criteria for one (or more) classic rheumatic diseases, but they do not need them. Sharp syndrome is distinguished from other overlapping syndromes by laboratory results: Sharp patients have rheumatic overlap syndrome plus anti-RNP antibodies [1].
Clinical Signs and Symptoms of Sharp Syndrome
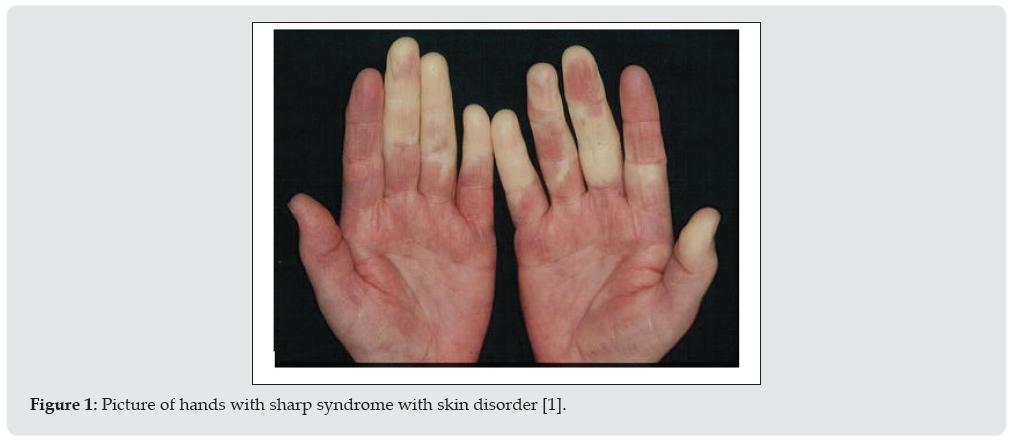
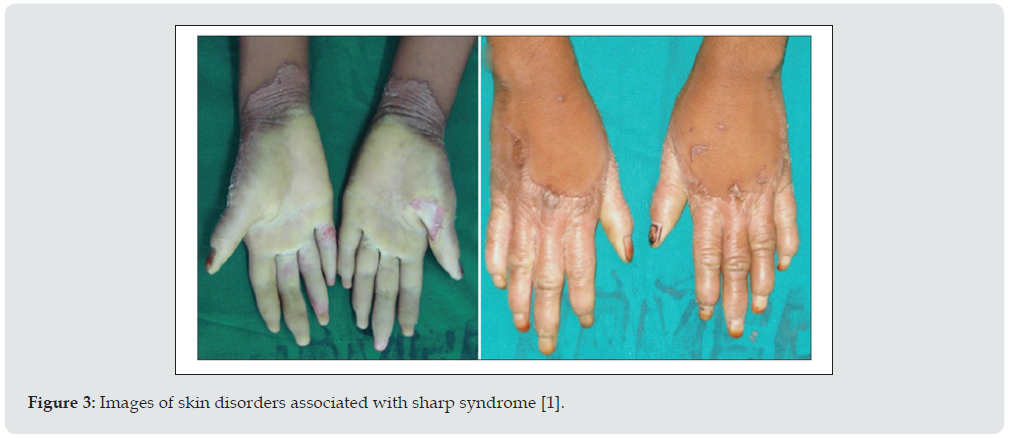
People with Sharp syndrome have symptoms that overlap with symptoms of two or more connective tissue diseases. These diseases include systemic lupus erythematosus, polymyositis, scleroderma, and rheumatoid arthritis. The condition known as Raynaud’s complication may precede additional symptoms of sharp syndrome. Raynaud’s complication, which is also seen in scleroderma, is characterized by the fingers and toes with cold pain and blue or white discoloration caused by spasm of blood vessels in the hands and feet in response to cold or stress. Tinnitus occurs in approximately 90% of people with Sharp Syndrome [1]. Multiple joint pain (polyarthritis) or arthritis can also occur in most people. Lupus-like skin inflammation is common in sun-exposed areas and hair loss, as well as skin sores on the fingers and face, such as those seen in scleroderma, are also seen in Sharp syndrome. Muscle weakness due to inflammation (myositis) of proximal muscle groups can also occur (Figure 2). Excessive recurrent symptoms include swelling and fatigue of the hands. Esophageal dysfunction occurs in at least half of people with Sharp syndrome. The esophagus is the tube that carries food from the mouth to the stomach. Esophageal problems often present with heartburn (gastric reflux) and difficulty swallowing solid foods. About half of people with Sharp Syndrome may normally have lung problems some time after the onset of the disease. Sharp syndrome lung disease can lead to respiratory problems caused by high blood pressure in the lungs (pulmonary hypertension) or inflammation of the lungs and ulcers in and around the air sacs (interstitial lung disease) [1,2].
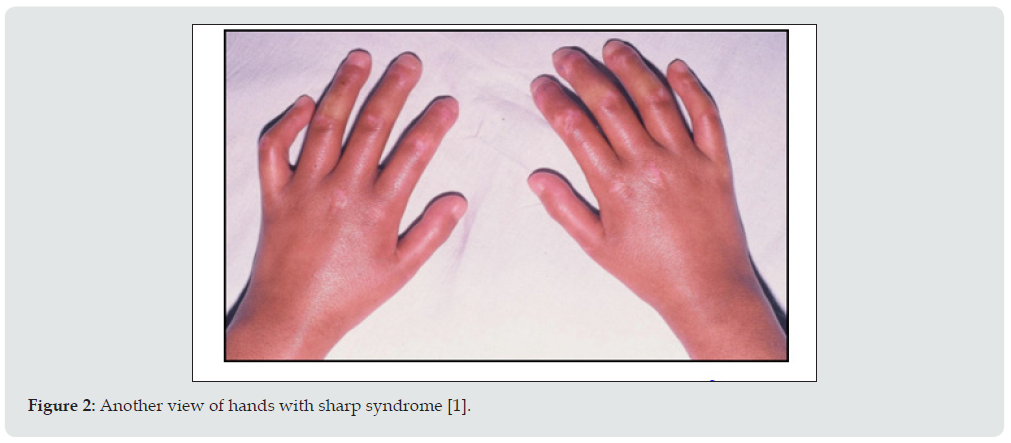
Heart involvement in Sharp syndrome is less common than lung problems, but can be serious if it does occur. Kidney disease occurs much less frequently in Sharp Syndrome than in lupus (10% of people with Sharp Syndrome) and is often mild in Sharp Syndrome. Neurological abnormalities occur in approximately 10% of people with Sharp syndrome [1,2]. Low levels of circulating red blood cells (anemia) and a decrease in the number of white blood cells (leukopenia) occur in 30 to 40% of cases. Lymph node disease (lymphadenopathy), spleen enlargement (splenomegaly), liver enlargement (hepatomegaly), and intestinal involvement may also occur in some cases [1,2]. Although medications may be needed to help control Sharp Syndrome, it has been reported that the disease eventually resolves in 40% of cases. Among patients with Sharp syndrome, organ targeting patterns have been reported that indicate subtypes of the disease. Some patients have more vascular manifestations, and have a higher risk for pulmonary hypertension. Some patients have more manifestations of myositis and are at higher risk for interstitial lung disease. Some patients with classic rheumatoid arthritis may have a lower risk of major internal organ damage [1,2]. Sharp syndrome is caused by immune reactions against the body (autoimmunity). The anti-RNP immune response that helps define the disease appears to mediate some of the damage caused by it. RNP molecules are usually found in the nucleus of all human cells, where they help produce messenger RNA that the immune system cannot find. However, in dead or dying cells, RNP molecules can be exposed to the immune system (Figure 3). Because RNP molecules in humans are almost identical to those in unicellular single-celled organisms, the human immune system can be deceived into thinking that RNP is like a dangerous attacker. The RNP gene is located in the short arm of chromosome 1 as 1p21.1. Several genes that control the immune system’s response to invaders and have the ability to hide or eliminate dead cell debris are also involved in Sharp syndrome. Previous exposure to other items that look like RNPs (such as previous viral infections) may also increase the risk. The additional effects of heredity and the environment on the risk of Sharp syndrome and on its manifestation and severity are likely [1,3].
The onset of Sharp Syndrome can occur at any time from early childhood to adulthood, but the average age of onset is 37 years. About 75% of people are women. The prevalence of Sharp syndrome in Norway is estimated to be 3.8 per 100,000 adults, and it appears to be similar in many other parts of the world, although the prevalence of Sharp syndrome is much higher in some ethnic groups, especially is in Japan [1,4]. The symptoms of the following disorders can be similar to the symptoms of Sharp Syndrome. A comparison may be useful for the differential diagnosis of this syndrome: Systemic lupus erythematosus (lupus) is a chronic inflammatory autoimmune disease that affects connective tissue. In autoimmune disorders, the body’s immune system attacks healthy cells and tissues, causing inflammation and dysfunction of various organ systems. In lupus, the organ systems are often involved in the skin, kidneys, blood and joints. Many different symptoms are associated with lupus, and most people do not experience all of the symptoms (Figure 4). In some cases, lupus may be a mild disorder that affects only a few organ systems. In other cases, it can lead to serious complications [1,5]. Scleroderma is an unusual autoimmune disease characterized by an unusual increase in the production and accumulation of collagen, the body’s main structural protein, in the skin and other organs. Systemic scleroderma is characterized by hardening (thickening) and thickening of the skin and abnormal changes in the destruction and formation of fibrous tissue (fibrosis) in specific organs of the body, including the lungs, heart, kidneys, and gastrointestinal tract. “Related symptoms, which may vary from case to case, may include abnormal discoloration and pain in the skin of the hands and feet due to exposure to cold temperatures (Raynaud’s complication), abnormal stenosis, thickening.” “Nail” and loss of skin elasticity, shortness of breath; Difficulty swallowing, muscle weakness; Joint pain; Heart abnormalities include irregular heartbeat (heartbeat), kidney abnormalities or other symptoms and findings [1,5]. Polymyositis is a rare autoimmune disease characterized by inflammatory changes in the destruction of muscle fibers and connective tissue supportive collagen. The main symptom of this disorder is muscle weakness, usually occurring in the neck, torso, shoulders and thighs. Eventually, it may be difficult to get up from a sitting position, climb stairs, lift objects, or reach the top of the head. Occasionally, joint pain and tenderness also occur. Additional symptoms may include pneumonia (interstitial pneumonitis and irregular heartbeat) [1,6]. Rheumatoid arthritis (RA) is a chronic prognosis of inflammatory arthritis that typically leads to joint destruction. Rheumatoid arthritis is characterized by inflammation of the joints (arthritis) on both sides of the body (symmetrical) leading to swelling, pain, and decreased mobility. The course of the disease is very variable[1,6].
Figure 4: Schematic of chromosome 1 where the RNP gene is located in the short arm of this chromosome as 1p21.1 [1].
Diagnosis of Sharp Syndrome
Sharp syndrome may be diagnosed based on a thorough clinical evaluation, accurate patient history, identification of characteristic findings, and specialized tests such as blood tests that show abnormally high levels of small nuclear nuclear ribonucleoprotein U1 (anti-RNP) [1,7].
Treatment Routes for Sharp Syndrome
The treatment of Sharp syndrome is based on the specific symptoms that are present in each case. Although no controlled studies have been performed in Sharp Syndrome, some patients with Sharp Syndrome have been included in previous trials for lupus, scleroderma, myositis, and rheumatoid arthritis. In general, these subgroups of Sharp syndrome appear to respond similarly to the same treatment as those reported in larger specific groups of rheumatic patients. These observations and clinical experiences accumulated by Sharp Syndrome specialists include the use of antimalarial drugs for the potential modulating effects of lupus-like disease, the use of vasodilators to treat Raynaud’s disease, the use of proton pump inhibitors for GERD, and the use of antidepressant drugs (Figure 5). Support additional diseases. Rheumatic drugs (DMARDs) are used for rheumatoid arthritis such as polyarthritis. Cohort studies in patients with Sharp Syndrome with pulmonary hypertension or other lung diseases have shown that these patients are likely to respond well to suppression of the invasive immune system compared to normal patients with similar lung disease for a variety of reasons [1,7]. Low to moderate doses of corticosteroids are often effective in rapidly controlling disease flares and may be used as part of long-term treatment in some patients, despite their significant long-term drug poisoning .[1,8] Nonsteroidal Anti- Inflammatory Drugs (NSAIDs) may also be used to help control mild inflammatory symptoms, although their use should be balanced with the risk of gastrointestinal complications. NSAIDs can rarely cause aseptic meningitis in some people. This seems to happen a little more often in patients with Sharp syndrome than in the other groups [1,8].
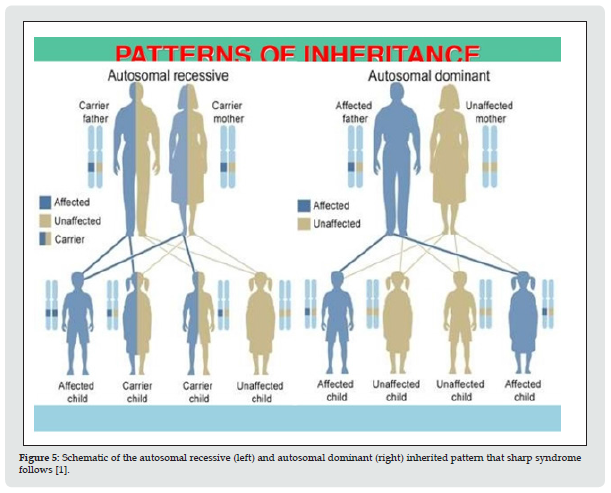
Figure 5: Schematic of the autosomal recessive (left) and autosomal dominant (right) inherited pattern that sharp syndrome follows [1].
Research Therapies
Research on Sharp syndrome is ongoing. Recent reports have identified promising strategies for treating mouse models of Sharp syndrome that should be followed up with human studies. Also, a large number of new anti-rheumatic drugs have recently been developed or are being developed to treat one or more classic rheumatic diseases. The main goal at this time is to better understand the mechanisms involved in the Sharp syndrome process. Discoveries like these can be a big step towards discovering a better cure or cure [1,8].
Discussion and Conclusion
Sharp syndrome, also known as Mixed Connective Tissue Disease (MTCD), is an uncommon systemic inflammatory rheumatic disease. Sharp syndrome is a specific subset of the broader category of rheumatic “overlapping syndromes,” a term used to describe when a patient has more than one characteristic of a classic rheumatic inflammatory disease. Sharp syndrome is caused by immune reactions against the body (autoimmunity). The anti-RNP immune response that helps define the disease appears to mediate some of the damage caused by it. RNP molecules are usually found in the nucleus of all human cells, where they help produce messenger RNA that the immune system cannot find. The treatment of Sharp syndrome is based on the specific symptoms that are present in each case. Although no controlled studies have been performed in Sharp Syndrome, some patients with Sharp Syndrome have been included in previous trials for lupus, scleroderma, myositis, and rheumatoid arthritis. In general, these subgroups of Sharp syndrome appear to respond similarly to the same treatment as those reported in larger specific groups of rheumatic patients [1-8].
References
- Asadi S (2021) Pathology in Medical Genetics Book, Amidi Publications, Iran 18.
- Zang Y, Martinez L, Fernandez I, Pignac-Kobinger J, Greidinger EL (2014) Conservation of Pathogenic TCR Homology across Class II Restrictions in Anti-Ribonucleoprotein Autoimmunity: Extended Efficacy of T Cell Vaccine Therapy. J Immunol 192(9): 4093-4102.
- Greidinger EL (2014) Mixed Connective Tissue Disease. Encyclopedia of Medical Immunology: Autoimmune Diseases, MacKay I, Rose NR (Eds.) Springer, New York , USA ISBN 13: 9780387848280.
- Carpintero MF, Martinez L, Fernandez I, Romero AC, Mejia C, et al. (2015) Diagnosis and risk stratification in patients with anti-RNP autoimmunity. Lupus 24(10): 1057-1066.
- Szodoray P, Hajas A, Kardos L, B Dezso, G Soos, et al. (2012) Distinct phenotypes in mixed connective tissue disease: subgroups and survival. Lupus 21(13): 1412-1422.
- Gunnarsson R, Molberg O, Gilboe IM, Gran JT (2011) PAHNOR1 Study Group. The prevalence and incidence of mixed connective tissue disease: a national multicentre survey of Norwegian patients. Ann Rheum Dis 70(6): 1047-1051.
- Maldonado M, Perez M, Pignac-Kobinger J, Triana E, Tozman E, et al. (2008) Clinical and Immunologic Manifestations of Mixed Connective Tissue Disease (MCTD) in Miami Population Compared to Midwestern Caucasian MCTD Population. J Rheumatol 35: 429-437.
- Greidinger EL (2018) Mixed Connective-Tissue Disease.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...