Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1381
Research Article(ISSN: 2644-1381) 
Predictors of the Duration of Management and Clinical Outcomes in Dogs with Acute Gastroenteritis Volume 3 - Issue 4
Felix Kundu Shima1*, Tersoo Uba2, Thaddaeus Ternenge Apaa3, Matthew Terzungwe Tion3, Temidayo Olutayo Omobowale1 and Helen Oyebukola Nottidge1
- 1Department of Veterinary Medicine, University of Ibadan, Nigeria
- 2Department of Statistics, Joseph Sarwuan Tarka University, Nigeria
- 3Department of Veterinary Medicine, Joseph Sarwuan Tarka University, Nigeria
Received: February 02, 2022 Published: February 14, 2022
*Corresponding author: Felix Kundu Shima, Department of Veterinary Medicine, University of Ibadan, Ibadan, Nigeria
DOI: 10.32474/CTBB.2022.03.000169
Abstract
Canine gastroenteritis represents one of the major presenting disorders in canine practice with challenges in its diagnosis and management. Its prognosis is reliant on the aetiology, severity of gastroenteritis, and response to treatment. Knowledge of factors influencing the course of this disorder is essential. This study evaluates prognosticators that can be utilised in predicting the clinical outcome and duration of management for dogs presenting with gastroenteritis. To achieve this, the clinical pathology profile of 104 dogs with gastroenteritis managed on outpatient was assayed using standard procedures. Data analysis was conducted using descriptive, Pearson’s correlation, and logistic regression model at α0.05 significance level. Haematological changes noted before treatment were anaemia, monocytopaenia, lymphocytosis, leukopaenia, neutropaenia, thrombocytopaenia, pancytopaenia, hyperfibrinogenaemia, and hypofibrinogenaemia. Biochemical changes were azotaemia, mild hyperglycaemia, hyperglobulinaemia, hyponatraemia, elevated albumin/globulin ratio, alanine aminotransferase, and alkaline phosphatase, hypercreatinaemia, hyperproteinaemia, hyperalbuminaemia, hypoalbuminaemia, hypokalaemia, hypercalcaemia, hypochloraemia, decreased aspartate aminotransferase and hypoproteinaemia. Reduction in the three blood cellular components ( pancytopaenia : OR = 0.2) or leukopaenia (OR = 3.5) prolonged duration of management by a day above the median duration of five days. Similarly, leukopaenia (OR = 0.1) , hypoalbuminaemia (OR = 7.1) , and colic (OR = 0.01) were associated with a poor prognosis. Leukopaenia was the only clinicopathologic abnormality that influenced both the duration of management and clinical outcome. The progression of gastroenteritis may not be determined by its cause but the presence of colic, hypoalbuminaemia (serum albumin conc. ≤ 20mg / dL) , leukopaenia (WBC ≤ 4,500 cells / L) , and pancytopaenia before treatment may predict clinical outcome and the duration of management for dogspresenting with gastroenteritis.
Keywords: Canine Practice; Sampling; Logistic Regression; Biomarkers; Prognosis; Outpatient.
Introduction
The functions of the digestive system include food digestion and absorption, toxins neutralisation and elimination of wastes products from the body. Gastroenteritis is one of the important disorders of the gastrointestinal tract which has caused high morbidity and several deaths of dogs in Nigeria [1-3]. Gastroenteritis represents one of the major presenting disorders in canine practice with challenges in its diagnosis and management [4]. The aetiologies are multifactorial and can be viral, bacterial, or protozoan, non-infectious, and systemic non-gastrointestinal diseases. Its management involves symptomatic treatment, rehydration, or prevention of dehydration, and/or management of secondary bacterial infections. Clinical pathological assessment is useful in establishing differential diagnoses, assessing response to treatment, and setting prognosis [5,6]. Good knowledge of the several biomarkers and predictors of the progression of a disease is relevant for establishing appropriate treatment and identification of patients that are critically ill [7]. Biomarkers are the several numbers of objective normal or abnormal findings observed from a patient under physical or clinical examination, indicating functional physiology or pathological status. Meaning clinicians must be able to repeatedly measure such clinical findings correctly and consistently [8].There are reports of marked alterations in the clinicopathologic indices of patients with gastroenteritis, with electrolyte imbalance and dehydration as the most common [5,9,10]. Expanding knowledge and effective prognostication can be beneficial in managing this disorder. This study was conducted to assess clinical pathologic profiles of Nigerian dogs with gastroenteritis, and their usefulness in diagnosis and prognostication. We hypothesized that regardless of the aetiology involved, changes in clinical pathology profiles of dogs with gastroenteritis at presentation are useful in predicting outcome and duration of management.
Ethical Considerations
This study received approval from the University of Ibadan Animal Care and Use Research Ethics Committee (protocol number UI – ACUREC / App / 03 / 2017 / 007) and consent from the dog owners and the management of the veterinary clinics sampled.
Materials and Methods
Sampling
A total of 104 client-owned dogs were sampled from five selected veterinary clinics in Nigeria, using a non-probability haphazard sampling method. Only cases with gastroenteritis that have not been treated before presentation, and were screened for Canine Parvovirus (CPV), Coronavirus (CCoV), Giardia, intestinal parasites were eligible for inclusion in the study. Demographic and qualitative data collected were breed, age, sex, husbandry practice, anaemia, anorexia, fever, depression/lethargy, dehydration, abdominal pains, body condition score, worming history, vaccination history, co-infection, diagnosis, faecal consistency (formed, watery, bloody, mucoid), frequency/duration of diarrhoea, frequency/duration of vomiting, the Duration of Management (DOM) and outcome (Death, Euthanasia, or Recovery). The DOM was defined as a difference in the number of days between first presentation and recovery or discharge. Qualitative data, blood samples for haematology and biochemical tests, as well as faecal samples, were collected once from each patient at initial presentation. The dogs were managed as out-patients and treatment were given based on the assessed need of each patient.
Laboratory Analyses
The dogs were screened first for CCoV, CPV, and Giardia using on-the-spot immunoassay kits (SensPERT® , VetAll Laboratories, Korea). Coprological techniques for laboratory screening of endoparasites were done as described by Urquhart et al. [11]. Blood samples for haematology and serum chemistry were analysed using commercial kits and standard procedures (Randox Laboratories Ltd, UK).
Data Handling and Statistical Analysis
The dogs (N =104) were grouped based on responses to treatment: survivors (n = 70) and non-survivors (n = 34) . To ascertain the strength of the associations and predictive ability of the covariates influencing the prognosis and the DOM (below or above the median duration), biochemical and haematological indices were coded as being either within or outside the normal reference limits. Also, qualitative variables were classified as either present or absent, vaccinated or nonvaccinated, dewormed or non-dewormed, viral, or other diseases, etc. The hematologic and biochemical variables were presented as a mean ± standard error of the mean. Descriptive statistics, Pearson’s correlation, and logistic regression were used in analysing the data. Pearson’s correlation was performed and variables demonstrating significant correlation with DOM and clinical outcomes were selected and further analysed by logistic regression model using the enter method. Hosmer-Lemeshow χ2 test, the omnibus test of model coefficients, and Nagelkerke’s pseudo-R2 were performed to ensure the final model was well fitted. All Statistical analyses were performed with SPSS (v20, IBM ) for Windows and evaluated at p < 0.05 significance level.
Results
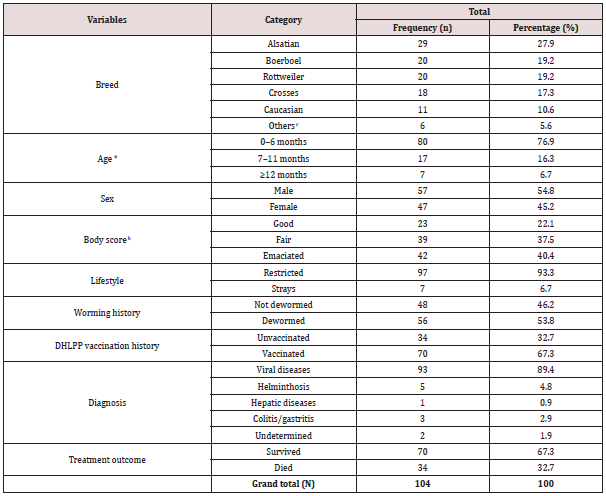
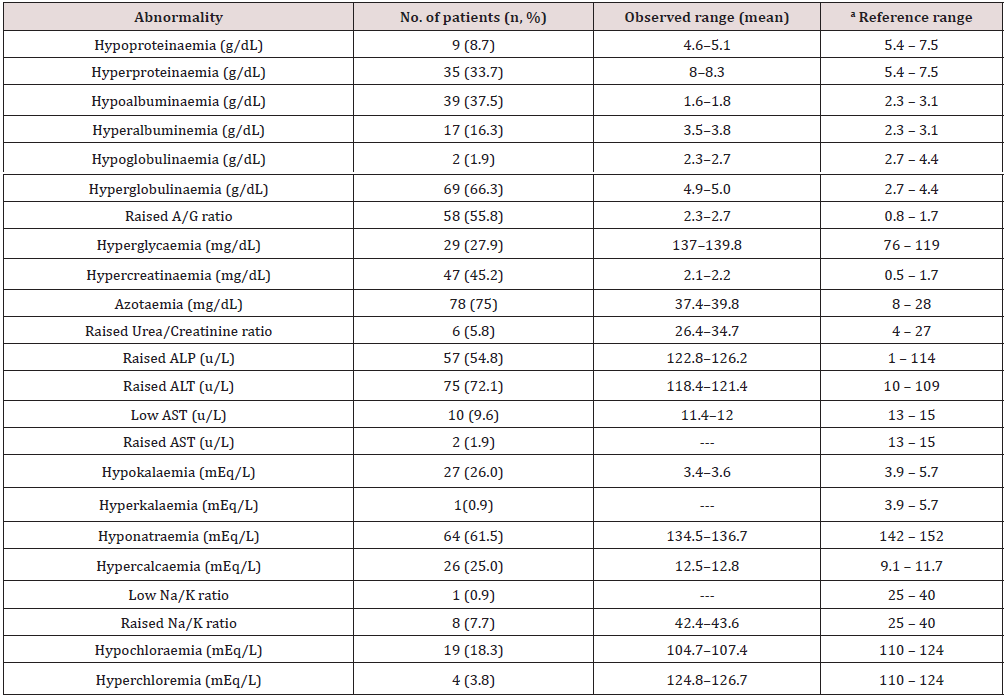
Table 1 summarises the characteristics of the sampled dogs. The patients (N =104) studied comprised of Alsatians (n1 = 29) , Boerboels (n2 = 20) , Rottweilers (n3 = 20) , Crossbreeds (n4 = 18) , Caucasia (n5 = 11) , and other least represented dog breeds (n6 = 6) . The dogs aged 7.7 months (95% CI = 5.7 – 9.7) . Eighty dogs were ≤6 months old and were mostly males (57) . The dogs had fair (39) and emaciated (42) body condition scores. Ninety-seven were restricted dogs, while 7 strayed. Gastroenteritis was more prevalent in restricted dogs (97 cases) than stray dogs (7 cases) . More than 50% of the dogs (56) were dewormed within the last 3 months before the illness. Diseases detected were majorly viral (93) and non-viral (11) . Two-third (70) of the patients survived, whereas one-third (34) died. Two-third (70) received at least a dose of the DHLPP vaccine, while one-third was unvaccinated. Clinicopathologic changes and variables influencing DOM and outcomes are presented in Tables 2–6.
aMean age of the dogs (7.7 months, median = 4 months, CI = 5.7–9.7).
bMean weight of the dogs (13.5kg, CI = 11.9–15.2); Average number of dogs per household (2.3±0.3; CI = 1.9–2.7).
cOthers (least represented breeds = Great Dane, Bullmastiff, Lhasa Apso, Pitbull, Doberman, and Indigenous).
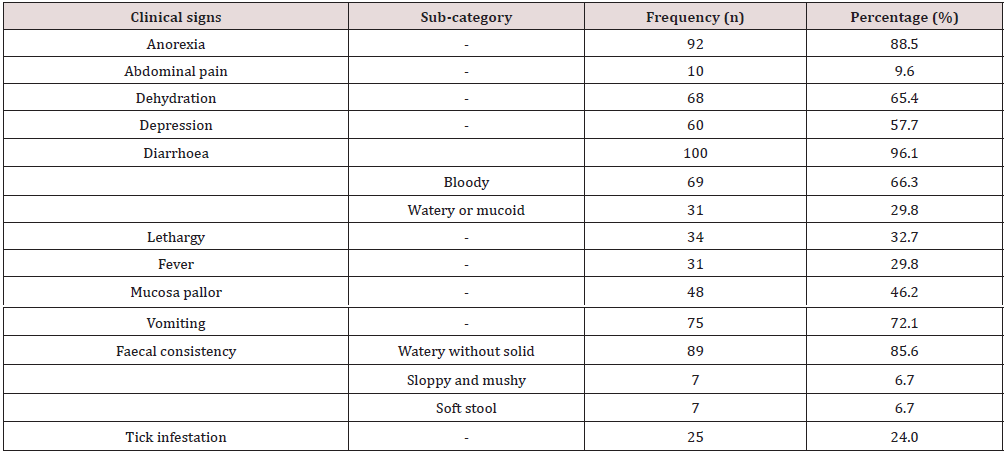
The mean rectal temperature (39.4°C, CI = 39.2-39.6°C); Mean respiratory rate (44.6 breaths/min, CI = 36.9–52.4); Mean heart rate (135.7±6.5 beats/ min, CI = 123-148.5); Mean diarrhoea episode (3.1±0.3, median = 2; CI = 2.6–3.6 times); Mean vomiting episodes (2.4±0.3, median = 1, CI = 3.0-1.8 times); Mean duration of diarrhoea (3.7±0.3, median = 3, CI = 3.2-4.2 days); Mean duration of clinic signs before presentation (3.3±0.3, median = 3, CI = 2.8-3.9 days); mean duration of management (5.3±0.3, median 5, CI = 4.8-5.9).
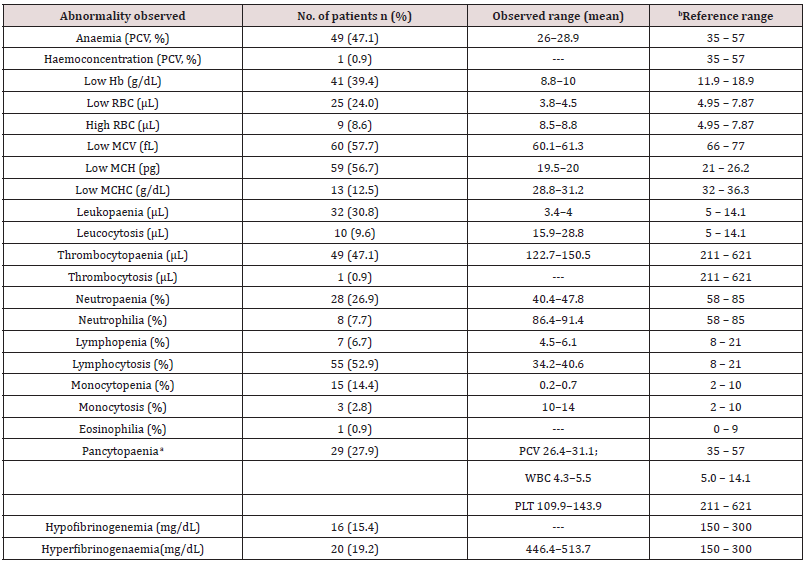
aPancytopaenia was defined as the combination of low haematocrit (PCV, <37% for dogs older than five months or <30% for dogs younger than five months), white blood cell counts (WBC, <6×103/L) and platelets (<200×103/L) counts [12].
bReference range [13].
Tp = total proteins; Alb = albumin; Glb = globulin; A/G ratio = albumin to globulin ratio; BUN = blood urea nitrogen; ALP = alkaline phosphatase, ALT = alanine aminotransferase; AST = aspartate transaminase; Crt. = creatinine; aReference range [13].
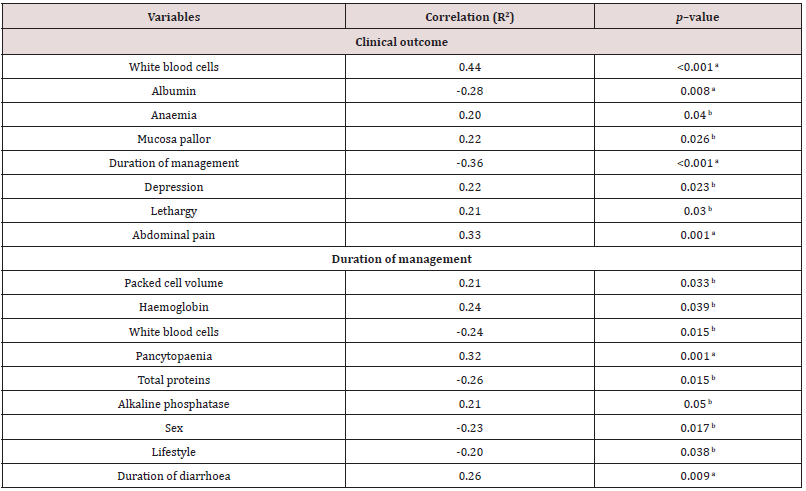
aCorrelation was significant at the 0.01 level.
bCorrelation was significant at the 0.05 level, N=104.
Table 6: Risk of poor prognosis and prolonged duration of management in the distinct groups compared to normal (OR =1).
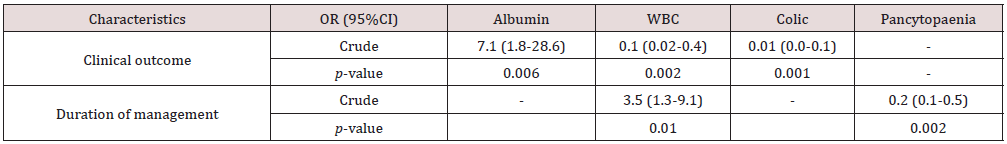
Note: CI = confidence interval. Changes in albumin concentration had increased likelihood with 7.1 times the odds of death for patients with hypoalbuminemia than patients without. Changes in total WBC and presence of colic had decreased likelihood and are 0.1 and 0.01 times respectively the odds of death for patients with leukopenia and colic than those without, all things being equal. Changes in WBC had increased likelihood with 3.5 times the odds of death for patients with leukopenia than patients without. The presence of pancytopenia had a decreased likelihood and has 0.2 times the odds of death for patients, all things being equal.
Discussion
This study assessed the importance of history taking, clinical examination, and clinical pathology profiles of dogs before treatment. This was to enable us to predict the duration of management and outcome in cases of canine gastroenteritis examined. Canine gastroenteritis was more prevalent in puppies below seven months, validating the report of Wells and Hepper [14]. Puppies are immunologically incompetent and maternallyderived antibodies wane early in life making them more vulnerable to infections [15]. The common clinical findings presented in Table 2 corroborate the reports of Castro, et al. [16]. Gastrointestinal inflammatory processes trigger anorexia, pyrexia, and weight loss by the release of inflammatory endogenous mediators, interleukin-1, and cachectin [17,18]. Haematologic changes recorded in Table 3 tally with findings from previous reports [6,19,20]. The microcytic hypochromic anaemia in this report may be linked to iron deficiency, following intestinal blood loss and inflammations from haemorrhagic gastroenteritis [21,22]. Neutrophilia may be due to immune system stimulation to control opportunistic bacteria during the onset of the disease. The later stages of viral infections are characterised by severe neutropaenia due to the destruction of immune cells and bacterial colonisation of the gut.
Biochemical responses recorded in the dog patients revealed that about 75% of them were azotaemic; comparable to existing findings [23]. This is attributable to dehydrating gastroenteritis and pre-renal uraemia with a reduction in glomeruli filtration rate or catabolic breakdown of tissues in pyretic animals [24]. The sampled dogs had a temperature range of 39.2–39.6℃, suggestive of pyrexia due to bacterial infections. The high rates of hyperglobulinaemia, hypoalbuminaemia, increased A:G ratio, hyperalbuminemia, and hypoproteinaemia observed corroborated previous reports [25,26]. Changes in total proteins were related to a marked reduction in dietary intake, malabsorption, and protein-losing enteropathy [25,26] hepatic synthesis of acute-phase proteins by damaged GI tissue and inflammation [27], destruction of the intestinal villi by infectious pathogens or systemic inflammatory response syndrome (SIRS)-mediated increased vascular permeability [28]. Increased A:G ratio can be equated to an increase in albumin with a relative decrease in globulin concentration. The hyperglycaemia reported here may reflect dehydration, stress, or ketosis that interfered with glucose utilization by the patients. This hyperglycaemia is transient and resolved following rehydration. This has been described in children suffering from severe gastroenteritis [29] and dogs with viral enteritis. Elevation in liver enzymes (ALP, ALT, BUN, and creatinine) concurs with previous reports [9,30]. These changes could be triggered by dehydration, hepatic hypoxia secondary to severe hypovolaemia or absorption of toxic wastes [30,31] or the young age of the sampled dogs [32].
Low levels of AST in 9.6% of the dogs sampled is unexplainable. However, malabsorption and malfunctioning or damaged liver prevented the release of enough quantities into circulation. The dogs sampled had mostly hyponatraemia, hypokalaemia, hypochloraemia, raised Na: K ratio, and hypercalcaemia, corroborating the findings of previous studies. Disturbances in these electrolytes have been linked to vomiting and diarrhoea or failure of the colon to conserve potassium by the patients [33]. Metabolic, systemic, and parasitic diseases such as hypoadrenocorticism, pancreatic disease, pyometra, renal diseases are the established causes of low Na: K ratio in dogs [34]. Hypercalcaemia may be relative to hyperalbuminemia or dehydrating gastroenteritis. Biochemical changes such as hypoproteinaemia, hypoalbuminaemia, hypoglycaemia, and hyperglycaemia are associated with interactions between multiple factors such as malnutrition, septicaemia, stress-induced activation of catecholamines, hypocalcaemia, hypokalaemia, hyponatraemia, hypochloraemia, hypomagnesaemia, azotaemia, liver damage induced by hypoperfusion and SIRS [6,35,36].
In the correlation analysis, total leukocyte counts, mucosae pallor, depression, lethargy, abdominal pain, and anaemia correlated with clinical outcome (Table 5). However, only changes in albumin, total WBC count, and colic had the strongest tendencies to influence prognosis (Table 6). The odds of death for dogs with hypoalbuminaemia (serum albumin conc. ≤20mg/dL), leukopaenia (WBC≤4,500 cells/L), and presence of colic were 7.1, 0.1, and 0.01 times, respectively the odds of death for patients without these changes. Leukopaenia and hypoalbuminaemia reported here tally with similar studies. The finding of leukopaenia as a biomarker of poor prognosis corroborates existing published reports [37,38].
Some researchers argued that neutrophils are more prognostic than leukocytes, when there is severe neutropaenia leukopaenia ensues, hence leukopaenia alone ought not to form the sole criterion of prognosis [37,39]. In contrast to that claim, severe neutropaenia was not found to be prognostic [40,41]. Contrary to the finding reported here and that of Mason et al. [39], Glickman, et al. [42] also reported that leukopaenia is not a biomarker of poor prognosis. The finding of hypoalbuminaemia as a biomarker of poor prognosis in this study concurs with the report of Boag [43]. Association of colic with poor prognosis may suggest the severity of the disease, intussusceptions, inflammation of the gut mucosae, or secondary infections. Besides the findings reported here, lymphopenia, vomiting, depression, body weight, changes in blood glucose, sodium, and AST concentrations [44] and how severe the disease is [45] determine the clinical outcome. Variables that correlated with DOM in this investigation included changes in PCV, WBC, Hb, pancytopaenia, total proteins, ALP, sex of the dog, lifestyle, and duration of diarrhoea (Table 7). The odds of pancytopaenia and leukopenia to prolong DOM in dogs with gastroenteritis by at least a day above the median five days were 0.2 and 3.5 times, respectively. In contrast, vomiting, depression, lymphopaenia, and hypoalbuminaemia were reported to prolong the duration of hospitalisation [6,46].
Table 7: Treatment options used in the management of the canine gastroenteritis cases studied indicating the drug used, dosage, frequency, route of administration, and percentage of patients who received a particular treatment.
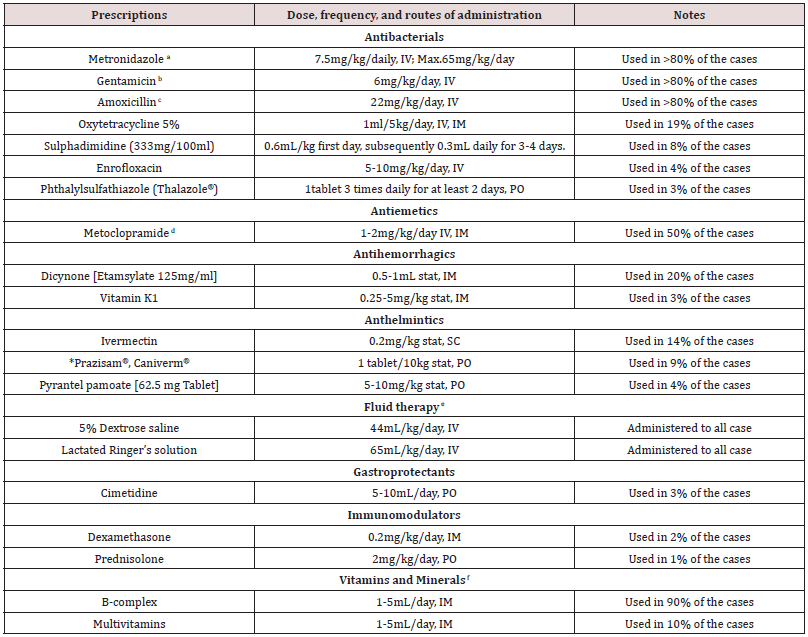
*NOTE-Prazisam®: Fixed-dose formulary of anthelminthics containing praziquantel 50mg, pyrantel pamoate 144mg, and fenbendazole 500mg.
Caniverm®: Praziquantel 50mg, pyrantel pamoate 144mg, and fenbendazole 150mg. The dogs were prescribed a, b, c, d, e, f extensively. The treatment options were decided by the clinicians based on the need of the individual case. The median duration of treatment from reporting to discharge was 5 days.
Leukopaenia was the only clinical pathology at admission that significantly influenced both clinical outcomes and the DOM as opposed to previous findings [6,42]. Also, pancytopaenia significantly increased the DOM as opposed to reports that it influences poor clinical outcomes in many infectious diseases [47]. The pancytopenia dogs studied improved with treatment, making pancytopaenia a nonsignificant prognosticator of poor outcomes. Ultimately, Goddard, et al. reported that an accurate prognosis may be obtained 24 hours postadmission. But this report showed that changes in total leukocyte counts (specifically leukopaenia), reduction in the three blood cellular components (pancytopaenia), albumin (particularly hypoalbuminaemia) and presence of colic at presentation are prognostic for poor outcomes and prolonged DOM in dogs with gastroenteritis. Interestingly, the type of disease is not a good predictor of clinical outcome and DOM in this study. These findings may assist clinicians in recognizing canine gastroenteritis candidates with substantial risk, and rapid selection of appropriate treatment and intensive care measures.
Acknowledgements
Colleagues, management of the veterinary clinics, as well as owners of the dogs are duly acknowledged for the consent and access granted to their facilities, animals, and samples collected.
References
- Shima FK, Mosugu JIT, Apaa TT (2014) Causes of mortality in dogs in and around Effurun/Warri Municipality of Delta State, Nigeria. Bulletin of Animal Health and Production in Africa 62: 387-396.
- Shima FK, Tion MT, Mosugu JI, Apaa TT (2015) Retrospective study of disease incidence and other clinical conditions diagnosed in owned dogs in Delta State, Nigeria, Journal of Advanced Veterinary and Animal Research 2: 435-449.
- Shima FK, Omotosho OO, Apaa TT, Omobowale TO, Nottidge HO (2021) A retrospective study of the prevalence of gastroenteritis in dogs attending some veterinary clinics in Nigeria. Rev Vét Clin 56: 170-176.
- Tams TR (2003) Gastrointestinal symptoms. Handbook of Small Animal Gastroenterology. TR Tams Ed. 2nd ed. Elsevier, USA.
- Goddard A, Leisewtz AL, Christopher MM, Duncan NM, Becker PJ (2008) Prognostic usefulness of blood leukocyte changes in canine parvoviral enteritis. Journal of Veterinary Internal Medicine 22: 309-316.
- Kalli I, Leontides LS, Mylonakis ME, Katerina Adamama-Moraitou, Timoleon Rallis, et al. (2010) Factors affecting the occurrence, duration of hospitalisation and final outcome in canine parvovirus infection. Research in Veterinary Science 89: 174-178.
- Schoeman JP, Goddard A, Leisewitz AL (2013) Biomarkers in canine parvovirus enteritis. New Zealand Veterinary Journal 61: 217-222.
- Strimbu K, Tavel JA (2010) What are biomarkers? Current Opinion in HIV and AIDS 5.6: 463-466.
- Salem NY (2014) Canine viral diarrhoea: clinical, haematologic and biochemical alterations with particular reference to in-clinic rapid diagnosis. Global Veterinaria 13(3): 302-307
- Kumar A, Kumar RK (2017) Clinico-hematobiochemical changes in parvoviral infection in dog. International Journal of Science, Environment and Technology 6: 2837-
- Urquhart GM, Duncan JL, Armour J, Dunn AM, Jennings FW (1996) Veterinary Parasitology. 2nd edn. Blackwell Science Ltd, Oxford, USA pp. 53-73.
- Weiss DJ, Evanson OA, Sykes J (1999) A retrospective study of canine pancytopenia. Veterinary Clinical Pathology 28: 83-88.
- Merck (2016) Reference guides. The Merck Veterinary Manual.
- Wells DL, Hepper PG (1999) Prevalence of disease in dogs purchased from an animal rescue shelter. Veterinary Records 144: 35-38.
- Jarret O, Ramsey I (2001) Vaccination. Manual of canine and feline infectious diseases. Ramsey I, Gloucester TB (Eds), British Small Animal Veterinary Association, The UK, pp. 41-50.
- Castro TX, Miranda SC, Labarthe NV, Silva LE, Cubel Garcia RCN (2007) Clinical and epidemiological aspects of canine parvovirus (CPV) Enteritis in the State of Rio de Janeiro: 1995-2004. Arquivo Brasileiro de Medicina Veterinariae Zootecnia 59: 333-339.
- Goddard A, Leisewitz AL (2010) Canine parvovirus. Veterinary Clinics of North America: Small Animal Practice 40: 1041-1053.
- Sharp CR, Declue AE, Haak CK, Honaker AR, Reinero CR (2010) Evaluation of the anti-endotoxin effects of polymyxin B in a feline model of endotoxaemia. Journal of Feline Medicine and Surgery 12: 278-285.
- Haligur M, Ozmen O, Sezer K, Sahinduran S (2009) Clinical, pathological and immunohistochemical findings in diarrheic dogs and evaluation of canine parvoviral and coronaviral enteritis. Journal of Animal and Veterinary Advances 8: 720-725
- Decaro N, Buonavoglia C (2012) Canine parvovirus - a review of epidemiological and diagnostic aspects, with emphasis on type 2c. Veterinary Microbiology 155: 1-12.
- Naigamwalla DZ, Webb JA, Giger U (2012) Iron deficiency anaemia. Canadian Veterinary Journal 53: 250-256.
- Torrente C, Manzanilla EG, Bosch L, Fresno L, Rivera Del Alamo M, et al. (2015) Plasma iron, C-reactive protein, albumin, and plasma fibrinogen concentrations in dogs with systemic inflammatory response syndrome. Journal of Veterinary Emergency and Critical Care 25: 611-619.
- Bhat AA, Wadhwa DR, Singh SP, Singh I (2013) Haematological and biochemical analysis in canine enteritis. Veterinary World 6: 380-383.
- Shinde SS, Rajguru DN, Anantwar LG, Mohd S, Ambore BN (2000) Clinicopathology and therapeutic management of canine gastroenteritis. The blue cross book 15: 26-29.
- Dossin O, Lavour R (2011) Protein-losing enteropathies in dogs. Veterinary Clinics of Small Animal 41: 399-418.
- Craven M, Caroline SM, Kenneth WS (2011) Granulomatous colitis of Boxer dogs. Veterinary Clinics of Small Animal 41: 433-445.
- Broek AH (1990) Serum protein electrophoresis in canine parvovirus enteritis. British Veterinary Journal 146: 255-259.
- Mazzaferro EM, Rudloff E, Kirby R (2002) The role of albumin replacement in the critically ill veterinary patient. Journal of Veterinary Emergency Critical Care 12: 113-124.
- Seth A, Aneja S (1995) Hyperglycemia in malnourished children with dehydrating gastroenteritis. Indian Journal of Paediatrics 62: 353-355.
- Shah SA, Sood NK, Wani N, Gupta K, Singh A (2013). Haemato-biochemical changes in canine parvoviral infection. Indian Journal of Veterinary Pathology 37: 131-133.
- Berghoff N, Steiner JM (2011) Laboratory tests for the diagnosis and management of chronic canine and feline enteropathies. Veterinary Clinics of Small Animal 41: 311-328.
- Macintire DK, Smith-Carr S (1997) Canine parvovirus. Part II. Clinical signs, diagnosis, and treatment. Compendium Continuing Education Practice for Veterinarians 19: 291-302
- Ettinger JS, Feldman EC (2010) Textbook of Veterinary Internal Medicine: Diseases of dog and cat. WB Saunders Company, Philadelphia, USA pp. 1310-1408.
- Roth L, Tyler RD (1999) Evaluation of low sodium: potassium ratios in dogs. Journal of Veterinary Diagnostic Investigation 11: 60-64.
- Li R, Humm KR (2015) Canine parvovirus infection. In: Silverstein DC, Hoper K (Eds). Small Animal Critical Care Medicine. 2nd Ed, St Louis, MO, USA Elsevier pp. 509-513.
- Mylonakis ME, Kalli I, Rallis TS (2016) Canine parvoviral enteritis: an update on the clinical diagnosis, treatment, and prevention. Veterinary Medicine: Research and Reports 7: 91-100.
- Potgieter LND, Jones JB, Patton CS, Webb-Martin TA (1981) Experimental parvovirus infection in dogs. Canadian Journal of Comparative Medicine 45: 212-216.
- O Sullivan G, Durham PJK, Smith JR, Campbell RSF (1984) Experimentally induced severe canine parvoviral enteritis. Australian Veterinary Journal 61: 1-4.
- Mason MJ, Gillett NA, Muggenburg BA (1987) Clinical, pathological, and epidemiological aspects of canine parvoviral enteritis in an unvaccinated closed beagle colony: 1978-1985. Journal of the American Animal Hospital Association 23: 183-192.
- Brunner C, Swango L (1985) Canine parvovirus infection: Effects on the immune system and factors that predispose to severe disease. Compendium on Continuing Education for the Practising Veterinarian 7: 979-989.
- McCaw DL, Harrington DP, Jones BD (1996) A retrospective study of canine parvovirus gastroenteritis: 89 cases. Journal of Veterinary Internal Medicine 10: 157.
- Glickman LT, Domanski LM, Patronek GJ, Visintainer F (1985) Breed-related risk factors for canine parvovirus enteritis. Journal of the American Veterinary Medical Association 187: 589-594.
- Boag A (2013) Managing parvovirus cases. In: 10th Emergency and Critical Care UK Annual Congress, pp: 200-207.
- Good JM, Otto CM (2006) Routine biomarkers of sepsis in dogs with canine parvoviral enteritis: a retrospective study (abstract). In: Proceedings of International Veterinary Emergency and Critical Care Symposium, pp: 8-9.
- Mantione NL, Otto CM (2005) Characterisation of the use of antiemetic agents in dogs with parvoviral enteritis treated at a veterinary teaching hospital: 77 cases (1997-2000). Journal of the American Veterinary Medical Association 227: 1787-1793.
- Schoeman JP, Van Schoor M (2008) Admission serum biochemical parameters are not associated with mortality in puppies with parvoviral diarrhoea (abstract). In: Proceeding’s 18th European College of Veterinary Internal Medicine-Companion Animal Congress, 4th-6th September 2008, Ghent, Belgium, Japn pp. 227-228.
- Frezoulis PS, Angelidou E, Karnezi D, Oikonomidis IL, Kritsepi-Konstantinou M, Kasabalis D, Melonakos ME (2017) Canine pancytopenia in a Mediterranean region: a retrospective study of 119 cases (2005-2013). Journal of Small Animal Practice 58(7): 395-402.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...