Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1381
Research Article(ISSN: 2644-1381) 
Predictors of Poor Prognosis in the Medium- Term of Traumatic Brain Injury Volume 1 - Issue 4
Noelia Rivas Martínez1*, Gustavo Rivas Martínez2, Sergio Eduardo Giannasi3, Nicolás Marcelo Ciarrocchi3
- Unidad de Cuidados Intensivos, Hospital de Trauma Manuel Giagni, Asunción Paraguay
- Departamento de Estadística e Investigación Operativa, Instituto Desarrollo, Paraguay
- Unidad de Cuidados Intensivos, Hospital Italiano de Buenos Aires, Argentina
Received: April 23, 2019; Published: May 02, 2019
*Corresponding author: Noelia Rivas Martínez, Unidad de Cuidados Intensivos, Hospital de Trauma Manuel Giagni, Asunción Paraguay
DOI: 10.32474/CTBB.2018.01.000116
Abstract
Objectives: The aims of this study are: a) to identify predictors of mid-term poor neurological outcome in Moderate or Severe Traumatic Brain Injury (MoSTBI); b) to recognize the consequences of MoSTBI, using neurological outcome and mortality rates six months after the patient´s discharge from the Intensive Care Unit (ICU).
Methods: Prospective, longitudinal study. Patients with MoSTBI admityted to the ICU of the Manuel Giagni Trauma Hospital (Paraguay) were included. Patient´s family members were contacted by phone six months after ICU discharge to assess survival and neurological outcome, using the Barthel Index.
Main measures: Demographics, Glasgow Coma Scale (GCS) on admission and at ICU discharge, New Injury Severity Score, APACHE II, Marshall CT Score, mechanical ventilation, sedation, ICU length of stay, the development of shock, infectious complications were collected.
Results: At six months after ICU discharge, mortality rate of MoSTBI was 43.9%, while and 57.60% of survivors achieved functional independence. The only predictor of poor neurological outcome was GCS at discharge from ICU (p-=0.003).
Conclusion: The GCS measured at ICU discharge was the best predictor of poor neurological outcome in MoSTBI patients. The measurement of this score once the patient has survived the acute phase could identify those most vulnerable and put in them a greater effort of rehabilitation.
Keywords: Traumatic Brain Injury; Predictors of Poor Prognosis; Disability; Functional Capacity; Mortality Rates; Barthel Index; Neurological Outcome
Introduction
Traumatic Brain Injury is the leading cause of death and disability around the world [1]. In the United States, around 2,5 million people visited emergency rooms suffering from TBI in 2013 and, among these, about 282.000 were hospitalized and 56.000 died [2]. In a study carried out in 25 European countries, TBI caused 37% of all trauma-related deaths; by extrapolating their results to the whole European population, the authors estimated 56.946 TBI-related deaths in 2012 only in the European Union [3]. The incidence of TBI, irrespective of its severity, shows stark geographic differences. Depending on the region, cases of TBI range from 60 cases per 100,000 inhabitants up to 12 times that number [4]. The incidence rate is higher in children, young adults and old people; and it is two or three times more common in men than women [5]. However, recent studies show that the average age for people with TBI is increasing, and that falls from heights are outpacing road traffic accident as the leading cause of TBI [6,7]. In addition to mortality rates, the consequences associated to TBI are measured considering how many years of productive life a person has lost and taking into account the various degrees of disability and deterioration of a person´s quality of life. Another consequence related to TBI is the economic burden it creates for patients, families and states [5]. For instance, in the United States, the direct and indirect cost of TBI in 2000 was estimated to be 110 billion USD [8].
The fatalities caused by TBI do not only occur immediately after the trauma or during the critical stage of hospitalization, but also months later. In a study carried out by Greenwald et al., people with moderate to severe TBI who went through rehabilitation were 2.2 times more prone to die than the general population of the United States of similar age, sex and race. In addition, their life expectancy decreased 6.6 years on average [9]. The group of TBI patients who were not able to follow the instructions when they entered rehabilitation was 6.9 more likely to die and their life expectancy decreased 12.2 years on average [9]. Furthermore, the long-term consequences of TBI are not only observed after a case of severe TBI, but also to an important degree in the cases previously classified as moderate or mild [4]. After discharge, a fair number of patients require a long rehabilitation process and can present long-term complications on a physical, cognitive and psychological level, which can impact their social, economic and work lives [4,10]. In Paraguay, injuries caused by all types of accidents were the fifth cause of death in 2014. Deaths caused by traffic accidents are estimated to be 20 per 100,000 inhabitants [11]. There are no data on the role of TBI on overall mortality nor on long-term neurological recovery coming from this country. It would be crucial to have precise, valid and trustworthy data in middle-income countries and, in general, from South America, in order to understand the impact of such an important public health issue on the population and potentially design TBI prevention strategies and identity research and education priorities. The aims of this study were therefore to report about survival and neurological outcome in patients suffering from Moderate or Severe Traumatic Brain Injury (MoSTBI) and to identify predictors of poor neurological outcome.
Methodology
This is a prospective, longitudinal and correlational study carried out in the Manuel Giagni Trauma Hospital (MGTH), the reference center for patients with trauma in Paraguay. This hospital depends on Ministry of Public Health and Social Welfare (MPH&SW). For this research, a database was developed. This database includes hospitalized patients with MoSTBI from October 1st, 2015 until September 30th, 2016. All the patients were in the Intensive Care Unit (Room A), which has 14 beds of high complexity.
Study Population
Hospitalized patients with MoSTBI according to the Glasgow Coma Scale (GCS) were included; the GCS was measured at the time of admission after initial resuscitation. In MGTH patients with TBI go to the emergency room, where they are evaluated by trauma surgeons and neurosurgeons. After the evaluation, doctors decide regarding surgical treatment, according to the injury displayed in the cranial computed tomography (CT scan). It is common that a decompressive craniectomy is performed (subject to the approval of the neurosurgeon on duty decides) in addition to hematoma drainage and placement of an external ventricular shunt catheter. After surgical treatment, patients enter ICU for neurocritical care monitoring. The management of patients with severe TBI in ICU includes: analgesia with continuous intravenous infusion of fetanyl; sedation with continuous intravenous infusion of midazolam for RASS -4; anticonvulsant prophylaxis with diphenylhydantoin in cases where the Glasgow Coma Scale is less than 8, intraparenchymal foreign body or cortical cerebral lesions; continuous monitoring of intracranial pressure (ICP) with ventricular shunt catheter. In the case of normal ICP and without adverse neurological changes, patients are re-evaluated at 48 hours, for the withdrawal of all neurocritical care measures. If the case of intracranial hypertension, cerebrospinal fluid drainage is performed and if there is no response, osmotic dilators are added (15% mannitol or 3% saline) along with an order for a new CT scan to reassess surgical treatment (decompressive craniectomy, drainage of new hematomas or expansions, extension of the previous decompressive craniectomy). In case the patient has not responded to all previous measures, a barbiturate-induced coma maybe evaluated. Patients who did not have a contact telephone number were excluded from the follow-up process that monitors their progress six months after discharge from ICU.
Study Variables
To identify the medium-term impact of MoSTBI, the patient´s family members were contacted in order to determine mortality rates and functional capacity. Functional capacity was evaluated by using the Barthel Index [12] validated to apply it over the phone [13] and categorized according to Shah et al. [14]. To analyze the variables related to neurological outcome the ranges taken into account were:
A. Good neurological outcome: Barthel Index 91-100 (including independent patients and patients with low dependency)
B. Poor neurological outcome: Barthel Index below 91
The time between the moment when the injury was caused, and hospital admission was taken into account in order to determine if time is a variable related to neurological outcome. Regarding infectious complications, all types of infections were considered. For instance: pneumonia caused by mechanical ventilation, ventriculitis or other infections detected by the medical staff and confirmed by bacteriology. To determine the presence of shock, the use of vasopressors was considered, regardless of dosage level. The categories of injuries were chosen considering the three most common causes; traffic accident, gunshot wound and falls from height. Injuries caused by other accidents were categorized as “others”. “Origin” is the place where the injuries were caused. This variable was divided in two categories; “central” which is the central province of Paraguay (where MGTH is located), and “country-side” which includes the rest of the country´s provinces.
Statistical Analysis
The sample comprises all the cases that match the criteria established for this study within the chosen time frame. During this study, some patients couldn’t be contacted. Therefore, in order to dismiss clinical differences between contacted and not contacted patients, some statistical tests were applied. The Mann-Whitney U test was applied for quantitative clinical characteristics and the Fisher´s exact test and Odds Ratio (OR) were applied for the dichotomous variables. On the statistically significant variables for neurological outcome, a logistic regression analysis was applied to quantify the probability of obtaining a poor neurological outcome in medium-term. A significance level of 0.05 is assumed for all the tests. The statistical software SPSS 20.00 was used to process data.
Ethical Considerations
The hospital ethics committee analyzed and approved the research protocol and the patient´s family members gave their consent.
Results
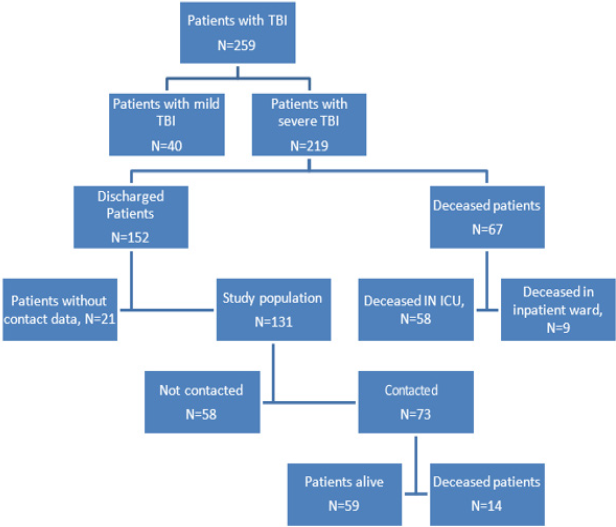
During this study period, 409 patients were admitted to the ICU. From the total of patients, 63% (259 patients) were admitted having a TBI diagnosis and among the TBI diagnosis 84% (219 patients) were cases of moderate or severe TBI. Figure 1 summarizes the process to reach the target population (Table 1).
Table 1: Demographic and clinical characteristics of the population studied.
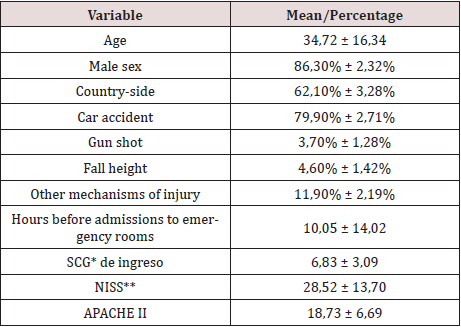
Glasgow Coma Scale
**New Injury Severity Score
Hospital mortality was 30.59% (67 patients, 58 in the ICU and 9 in the inpatient ward, Figure 1). Mortality rate for moderate TBI was 22.58% and for severe TBI 31.91%. Mortality rates of moderate TBI vs severe TBI do not show significant statistical differences (p-value 0.401). Therefore, cases of moderate and severe TBI were put in the same group to study the risk factors of hospital mortality. Table 2 illustrates the risk factors of hospital mortality. The APACHE score II (p-value 0.001), Marshall CT score (p-value 0.001), days in ICU (p-value 0.001), gender (p-value 0.018), and presence of shock (p-value 0.002) are statistically significant variables for risk of hospital mortality. During this study, some patients couldn’t be contacted. Therefore, in order to dismiss clinical differences between contacted and not contacted patients, some statistical tests were applied. The Mann-Whitney U test and Fisher´s exact test was used to determine if differences regarding clinical feature and severity during hospitalization in ICU exist between contacted patients and not contacted patients. In all the cases, the p-value associated with the statistical test was under 0.05. Consequently, the patients who were contacted can be assumed to have similar features to those not contacted. Mortality rates six months after discharge from ICU were 19.18% (14 patients out of 73 patients contacted over the phone, see Figure 1). Taking into account the similarities between contacted and not contacted patients, the estimated mortality rate for all discharged patients is 19.18%. Considering mortality rates six months after discharge and hospital mortality, the overall mortality rate of severe TBI after six months is 43.90%.
As well as with patients admitted for MoSTBI, the clinical variables between “alive and dead” during hospitalization in ICU were compared in patients contacted after six months. These clinical variables were compared to recognize the risk factors of mortality six months after discharge from ICU (Table 3). Risk factors for mortality six months after discharge from ICU were: Age upon admission (p-value 0.021), APACHE II score (p-value 0.044), days of sedation (p-value 0.027), days on mechanical ventilation (p-value 0.001), days in ICU (p-value 0.001) and Glasgow Coma Scale at discharge from ICU (p-value 0.001) (Table 3). The functional capacity six months after discharge according to the Barthel index showed that 57.60% (34 patients) reached functional independence, 5.10% (3 patients) scarce dependence, 11.90% (7 patients) moderate dependence, 13.60% (8 patients) severe dependence and 11.90% (7 patients) total dependence.
Table 3: Risk factors for mortality at 6 six months after discharge from ICU.
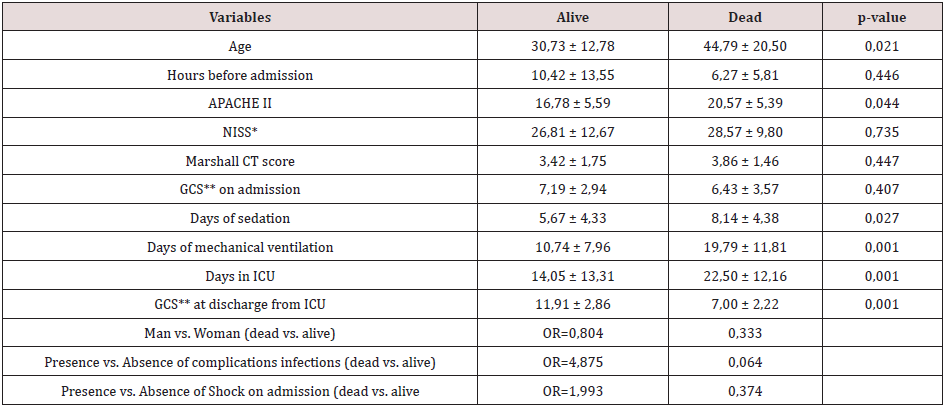
*New Injury Severity Score
**Glasgow Coma Scale
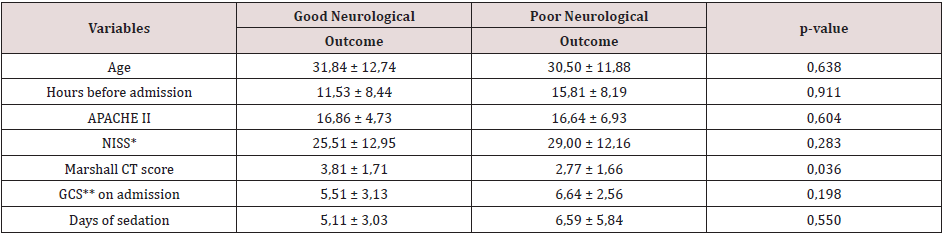
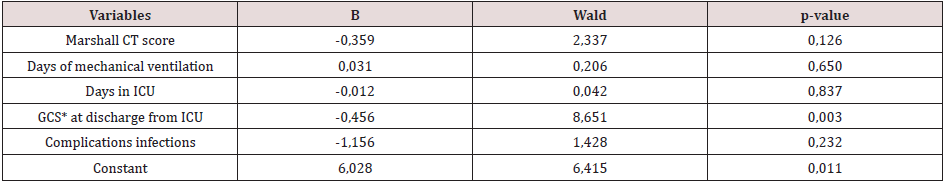
The clinical characteristics associated with a neurological outcome six months after discharge from ICU (Table 4) were: Marshall CT score (p-value 0.036), days on mechanical ventilation (p-value 0.011), days in ICU (p-value 0.016), GCS at discharge from ICU (p-value 0.001) and infectious complications (p-value 0.014). On the statistically significant risk factors for neurological outcome in the medium-term, a logistic regression analysis has been applied. As a result, the GCS at discharge from the ICU was the only variable with statistical significance to predict poor neurological prognosis (p-value 0.003) (Table 5). Therefore, it was decided to build a logistic regression model with GCS at discharge from ICU, obtaining the following mathematical model to estimate the poor neurological outcome in the medium-term.
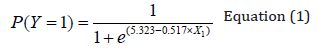
In equation (1), P (I = 1) is the probability of obtaining a poor neurological outcome according to GCS at discharge from ICU (X1) and e represents the base of the Neperian logarithm approximately equal to 2,71828.
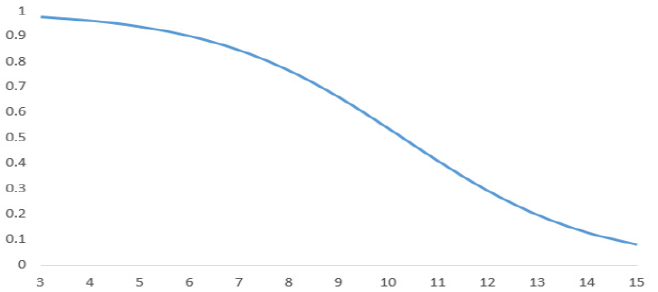
Applying the proposed model in equation (1), the following estimated probabilities of poor neurological outcome are obtained for the different GCS values at discharge from ICU: GCS at discharge from ICU 3: 0.978 probabilities of poor neurological outcome, for 4: 0.963, 5: 0.939, 6: 0.902, 7: 0.846, 8: 0.766, 9: 0.662, 10: 0.538, 11: 0.410, 12: 0.293, 13: 0.198, 14: 0.128 and 15: 0.081 (Figure 2). The goodness-of-fit of the proposed model is moderate, around 36% obtained from the Nagelkerke R2.
Discussion
Manuel Giagni Trauma Hospital is the only public medical center specialized in trauma in Paraguay. As a result, it deals with most of the polytraumatized patients in the country. This is also noticeable because of the high number of patients they receive from the country side in despite of long distances. Therefore, the study population can be considered representative at a country level. The study population is characterized for being mostly composed of male sex and young. Traffic accidents are the main cause of injury, matching the world trend [5,15]. Age on admission is a risk factor for mortality six months after discharge, the risk is greater at older age, also described by other authors as a prognostic factor in the acute phase of trauma [1,16], as well, as a functional prognostic factor [17]. However, in our study, functional capacity is not affected by age. Probably, mortality is dependent on the patient´s biological reserve; on the other hand the neurological outcome depends on the neurological damage suffered.
According to this study, women have a higher risk of hospital mortality, which is an interesting research topic. However, it is not possible to explain this phenomenon with this study due to the sample size of this gender. At six months, gender does not represent a risk factor of mortality. Hospital mortality of moderate TBI was 22.58% and 31.91% for severe TBI, these results are similar to other studies [2,3,4,18]. In our study, the mortality rate of MoSTBI after six months was 43.90%, these results are similar to the results of the medical treatment group “RESCUEicp Trial Collaborators” which got a 48.9% mortality rate of severe TBI after six months [15]. However, in the group of surgical treatment, mortality after six months was 26.9%. Despite the fact that decompressive craniectomy was performed upon admission to the emergency room, in our study population, the gap between our results and RESCUEicp Trial Collaborators´ results could be explained by the time it takes to arrive at MGTH (average hours 10.05 ± 14.02).
The patients who died in the hospital had fewer average days in ICU and did not display statistically significant differences in the average days of sedation and mechanical ventilation. However, in the population discharged from the hospital, those who died had, on average, more days of sedation, mechanical ventilation and length of stay in ICU. This could indicate that patients with MoSTBI die in the hospital and not because of the complications of hospitalization. However, in the medium-term (six months after discharge from the ICU) the effect of complications during hospitalization (in ICU) is perceived in mortality rates. The fact that the Marshall CT Score (p-value 0.001) is higher in those who died in the hospital, also supports this conjecture.
It is striking that the GCS on admission is not a risk factor for hospital mortality, not even for mortality six months after discharge. This could be due to multiple factors, such as the long time it takes to arrive to the emergency room for most patients (average hours before admission 10.05 ± 14.02) and the implications that this entails, such as previous intubation, sedation, among others. However, the GCS measured at discharge from the ICU represented a risk factor for mortality after six months, being higher in those who survived. As expected, the APACHE II score was a negative prognostic factor for mortality during hospitalization and at six months. Likewise, the presence of shock at admission was associated with higher hospital mortality [19].
The consequence in the medium-term of the MoSTBI, it was measured with the maximum functional capacity attained after six months of ICU discharge (as measured by the Barthel Index). The results were: 57.60% reach independence, an important percentage shows different degrees of dependence, even with a total and severe dependency level of 25.5%. For moderate or severe TBI in the sub-acute phase (54 ± 24 days post injury) Sandhaug et al. [20] described that 57% of patients with moderate TBI and 91% of patients with severe TBI remained inactive, even 24% of patients with severe TBI had severe disability. The RESCUEicp group used the Glasgow Outcome Scale Extended (GOSE) to assess the neurological outcome at six months. According to their findings, 30.4% of patients in the surgical treatment group and 16, 5% of patients in the medical treatment group had severe dependence (vegetative state and dependence on others for care). They also found that in 42.8% of patients in the surgical treatment group and 34.6% of the patients in the medical treatment group showed positive neurological outcome (independence in the home, moderate disability and good recovery) [15]. It should be noted that although our results were similar to those found in these studies, none of our patients had formal rehabilitation support at discharge from the hospital.
For Sandhaug et al. [20], the GCS at admission to rehabilitation, the total score at the functional level at admission to rehabilitation, the length of stay in the rehabilitation unit and the duration of posttraumatic amnesia were predictors of functional level upon discharge of rehabilitation. In our study, we evaluated the clinical variables of severity during hospitalization based on the results at six months after discharge from the ICU. After assessing these clinical variables, we determined that patients with plus average days on mechanical ventilation, length of stay in ICU, GCS at discharge from ICU and infectious complications, presented worse neurological outcomes. On the other hand, we observed that patients with the worst neurological outcomes were associated with a lower Marshall score. We found no theoretical explanations for this result, so we can propose another study with a larger sample size to further study this phenomenon. After the logistic regression analysis, GCS at discharge from ICU was identified as a predictor of poor neurological outcome in our studied population. With just one variable the predicted capacity is moderate. To improve this predicted capacity, other clinical variables should be considered during the patient’s internment in the ICU. This study should then be replicated. Although, with the proposed model it is possible to estimate the probabilities of poor neurological prognosis at medium-term without the necessity of conducting a follow-up of the patients. This implies a very important simplification of costs and other complications that the follow up of patient required.
It is important to bear in mind that the functional capacities assessed by the Barthel index are very basic in a person´s daily life. Some of these activities could be as elementary as eating, moving between chair and bed, washing oneself, bowel control, etc. Although these activities are essential, they do not cover all the aspects of living a full and productive life, such as working and relating with other people. Kamal et al. In a study conducted in a trauma center in India, they describe that after six months only 15.72% of patients had a recovery considered good [21]. Colanatonio et al. in a study to follow-up what happens to patients with moderate to severe TBI up to 24 years after, found that only 29% of patients were working full-time, whereas those who were more limited in activities such as money management and shopping had significant relationships between the limitations of cognitive activity and residual deterioration in follow- up [22]. This type of work, as well as the evaluation of the consequences on families and the society is still pending research in Paraguay.
Limitations of this Research
The main limitation of this research was the inability to followup all the cases. Currently, actions are in place to deal with this issue. However, social, cultural and economic characteristics of Paraguay make it difficult to contact the patients for follow-up. Additionally, it is important to put in place a personalized follow-up of patients in order to analyze indicators related to cognitive, behavioral and social life capabilities in addition to physical abilities.
Conclusion
The GCS measured at discharge from ICU is a predictor of poor neurological outcome in the medium-term of TBI. The measurement of this score once the patient has survived the acute phase could identify those most vulnerable and put in them a greater effort of rehabilitation.
References
- Stocchetti N, Carbonara M, Citerio G, Ercole A, Skrifvars MB (2017) Severe traumatic brain injury: targeted management in the intensive care unit. The Lancet Neurology 16(6): 452-464.
- Taylor CA, Bell JM, Breiding MJ, Xu L (2017) Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR Surveill Summ 66(No. SS-9):1-16.
- Majdan M, Plancikova D, Brazinova A, Rusnak M (2016) Epidemiology of traumatic brain injuries in Europe: a cross-sectional analysis. The Lancet Public Health 1(2): 76-83.
- Stocchetti N, Zaniera E (2016) Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Critical Care 20:148.
- World Health Organization: Neurological Disorders: Public Health Challenges. 3.10 Traumatic brain injuries.
- Roozenbeek B, Maas AI, Menon DK (2013) Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol 9(4): 231-236.
- Stocchetti N, Maas A IR (2014) Traumatic Intracranial Hypertension. N Engl J Med 370: 2121- 2130.
- Graves JM, Sears JM, Vavilala MS, Rivara FP (2013) The burden of traumatic brain injury among adolescent and young adult workers in Washington State. Journal of Safety Research 45: 133-139.
- Greenwald BD, Hammond FM, Harrison-Felix C (2015) Mortality following traumatic brain injury among individuals unable to follow commands at the time of rehabilitation admission: A National Institute on Disability and Rehabilitation Research traumatic brain injury model systems study. J Neurotrauma 32: 1883-1892.
- Simpson G, Pfeiffer D, Keogh S, Lane B (2016) Describing an Early Social Work Intervention Program for Families after Severe Traumatic Brain Injury. J Soc Work Disabil Rehabil 15: 3-4.
- http://mspbs.gov.py/dvent/boletin-epidemiologico-ent-2015/
- Cid-Ruzafa J, Moreno J (1997) VALORACIÓN DE LA DISCAPACIDAD FÍSICA: EL INDICE DE BARTHEL Revista Española de Salud Pública 71(2): 127-137.
- Korner-Bitensky N, Wood-Dauphinee S (1995) Barthel Index information elicited over the telephone. Is it reliable? Am J Phys Med Rehabil 74(1): 9-18.
- Shah S, Vanclay F, Cooper B (1989) Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol 42(8): 703-709.
- Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA (2016) for the RESCUEicp Trial Collaborators. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. N Engl J Med 375(12): 1119-1130.
- Dagher JH, Habra N, Lamoureux J, De Guise E, Feyz M (2010) Global outcome in acute phase of treatment following moderate-to-severe traumatic brain injury from motor vehicle collisions vs assaults. Brain Inj 24(12): 1389-1398.
- Taw BB, Lam AC, Ho FL, Hung KN (2012) Functional survival after acute care for severe head injury at a designated trauma center in Hong Kong. Asian J Surg 35(3): 117-122.
- Rosenfeld JV, Maas AI, Bragge P, Morganti-Kossmann MC (2012) Early management of severe traumatic brain injury. Lancet 380: 1088-1098.
- Teasdale G, Jennett B (1976) Assessment and prognosis of coma after head injury. Acta Neurochir 34: 45-55.
- Sandhaug M, Andelic N, Vatne A, Seiler S (2010) Functional level during sub-acute rehabilitation after traumatic brain injury: course and predictors of outcome. Brain Inj 24(5): 740-747.
- Kamal VK, Agrawal D, Pandey RM (2016) Epidemiology, clinical characteristics and outcomes of traumatic brain injury: Evidences from integrated level 1 trauma center in India. J Neurosci RuralPract 7(4): 515.
- Colantonio A, Ratcliff G, Chase S, Kelsey S (2004) Long-term outcomes after moderate to severe traumatic brain injury. Disabil Rehabil 26(5): 253-261.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...