Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1381
Research Article(ISSN: 2644-1381) 
Evaluation of D-amino Acid in Combination with Flucloxacillin on the Formation and Dispersal of Staphylococcus aureus Biofilm Volume 1 - Issue 5
Khalid Faisal Alahmadi1, Abdulrahman Mohammed Alalyani1, Salman Ali Abdali1, Emad Alharbi1, Abdulaziz Jafar almusallam1, Khalid Ibrahim Alahmadi2 and Ammar AL-Farga3*
- 1King Fahad Hospital, Ministry of Health, Al Madinah AI Munawwarah, Saudi Arabia
- 2Almeeqat Hospital, Ministry of Health, Al Madinah AI Munawwarah, Saudi Arabia
- 3Biochemistry Department, College of Sciences, University of Jeddah, Saudi Arabia
Received: October 10, 2019; Published: October 16, 2019
*Corresponding author: Ammar AL-Farga, Biochemistry Department, College of Sciences, University of Jeddah, Saudi Arabia
DOI: 10.32474/CTBB.2018.01.000123
Abstract
Staphylococcus aureus is one of the most common bacterial strainsand has been linked to various infections ranging from light respiratory and skin infections to fatal conditions such as endocarditis osteomyelitis and periodontitis. However, S. aureus has been resistant to antibacterial drugs and body immune system response mechanisms owing to its efficient adaptation mechanisms such as biofilm formations. D-Amino acids are believed to play a significant role in the structural formation of the biofilm’s peptidoglycan. The amino acids form the peptide chains and crosslinks with N-acetylmuramic acid. Altering the structural composition of the compounds would compromise the mechanical integrity of the biofilm and, thus, could be used as a potential target for preventing biofilm formation. The aim of the given study is to evaluate the effect of D-amino acid in combination with flucloxacillin on the formation and dispersal of Staphylococcus aureus biofilm. This is achieved by determining the minimum inhibitory and lethal concentrations of D-Amino acids (D-glutamate, D-aspartate and D-methionine) in combination with flucloxacillin against Staphylococcus aureus. The effectiveness of D-Amino acids and flucloxacillin is investigated separately and the results compared. D-aspartate and D-methionine show antibacterial activity whereas D-Glutamate promotes bacterium multiplication. The minimum lethal concentration of flucloxacillin is 1.25mg/mL, whereas the minimum inhibitory concentration was 0.625mg/mL. D-methionine improved the flucloxacillin’s antibacterial efficacy and demonstrated a potential clinical significance by ANOVA test.
Keywords: D-amino acid;Staphylococcus aureus;Flucloxacillin
Introduction
Staphylococcus aureus (Staph aureus, S. aureus or Golden staph) is a Gram-positive bacterium that belongs to the phylum Firmicutes(Marking & Shaw). The bacterium is non-motile, spherical shaped and grows in clusters that resemble a bunch of grapes.When grown on blood agar plate, S. aureus forms round golden-yellow colonies with haemolysis. Staphylococcus aureus is facultative-anaerobic, catalase and coagulase positive and, therefore, can be isolated from other Staphylococci by a coagulase test. Staph aureus is a human commensal parasite that has been identified to colonise the anterior nares of about 30-50% of people. The bacterium commonly grows in the respiratory tract, particularly the nasal cavity and the skin. However, Golden staph has been identified with several respiratory and skin infections (Hood, 2015)[1]. The bacterium is said to be an opportunistic microgram that tends to cause infections due to several factors such as a weakened immune system.
The type of infections that arise from S. aureus range from simple skin lesions, boils and sties to more serious infections such as endocarditis [2], steomyelitis (Tang, et al., 2017), periodontitis and peri-implantitis [3] and fatal septic shock meningitis and pneumonia Jansen (Van Vuuren, 2015). Like all other pathogens, it has evolved mechanisms to establish itself in the host and cause infection by means of virulence factors. These include surface proteins that help bacterium attach to the host cell and prevent phagocytosis. Invasions such as kinases and hyaluronidase are employed to help the spread of the bacterium. In addition, biochemical processes and toxins enhance survival [4]. Additionally, S.aureussecretes agr-controlled virulence factors such as immune avoidance and toxic antibodies that lyse inflammatory effectors of the immune system such as polymorphonuclear leukocytes (PMNs).
Staphylococcus aureus Antibiotic Resistance Mechanism
Staphylococcus aureus is the leading cause of many bacterial infections in the world.According to Pantosti et al. (2007), the bacterium quickly evolves into multidrug-resistant clones upon invading the body. The transfer of this antibiotic resistance trait within the S. aureus colony can be linked to multiple gene transfer mechanisms. Many of the strains found in hospitals are often resistant to many antibiotics. In fact, vancomycin intermediate resistant strains have also been reported [5]Methicillin resistance Staphylococcus aureus(MRSA) is the most common and widespread resistant strain. MRSA is resistant to antiseptics and disinfectants, thereby aiding its survival in the hospital environment [6].
Several mechanisms have been deployed by the bacterium to acquire resistance such as by acquisition of extrachromosomal plasmids or additional genetic information in the chromosome and by mutations in chromosomal genes [6]. In the biofilm environment, S. aureus can transfer genes through conjugation and mobilization (Savage et al. 2013). The bacteria also have an increased rate of bacteriophage release and, hence, the transfer can take place through transduction (Resch et al., 2005). Some scientific experiments have observed that mobile genetic elements that are transferred primarily between the bacteria consisting of the antibiotic resistance genes might be responsible for the formation of biofilm as well [7,8]. Notably, S. aureus developed a strain that hinders the body’s immune response mechanism of iron starvation by obtaining the supply from transferrin and heme using alphahemolysin toxins (Dahners, 2015). The toxin forms pores on red blood cells to access hemoproteins. The bacterium also develops circumvention mechanisms to obtain other ions such as copper and manganese from the blood cells.
The current medical response to the growing clinical complexity of bacterium infections includes the application of carbolic acid, typically, chlorhexadine and mupirocin [9]. An attempt to supplement the conventional treatment by triggering the host’s immune system and the microbial virulence factors is currently under study. Dryden et al. [10] report a prospect vaccination solution to the outmaneuvering antibiotics resistant bacterium’s strain as a permanent solution to S. aureus’ antibiotic resistance adaptation.
Staphylococcus aureus Biofilms Mechanism
A biofilm is defined as an assemblage of microbial cells that is irreversibly associated with a surface and enclosed in a matrix of primarily polysaccharide material (Donlan, 2002). Biofilms are a bacterial community embedded in a matrix produced from polysaccharides, protein and DNA and attached to a tissue surface. The matrix of bacterial biofilms is responsible for structural stability and prime protection of the group.S. aureus’ consortia have shown significant resistance to antibiotics, biocides and all other chemical toxicants (Høiby et al., 2010). In addition, the matrix can resist the human body’s immune system and is not susceptible to phagocytosis contrary to planktonic growth.
The development of S. aureus’biofilm typically proceeds through five stages: attachment, multiplication, exodus, maturation and dispersal (Figure1). During the attachment phase, planktonic bacterium attaches to the surface of its host tissue. Throughout the process, the cells utilize microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) for cell-to-cell attachment and bind to the host matrix components. The multiplication stage begins with asexual reproduction. The cells undergo multiple cell divisions and accumulate, thereby forming the cluster. In the process, the bacteria release extracellular proteins (FnBPs, ClfB and SDRC) and polysaccharide intracellular adhesin (PIA) that promote cellular binding at the initial stages of the attachment and maintain the stability of the immature biofilm. Cytoplasmic nucleoid-associated proteins bind to eDNA and form a component of the extracellular matrix (ECM).
By the end of the multiplication stage, a specific and coordinated bacterium cells’ release is observed. This controlled cell movement marks the exodus phase. The process typically begins six hours after the bacterium’s attachment and coincides with microcolony formation. The detachment is mediated by nuclease-initiated eDNA degradation. This stage is then followed by the development of a micro-colony structure. Other than increased surface area, the structure also provides the site for nutrient exchange and waste removal. High-density biofilm develops to adapt to the environmental stressor. These processes mark the maturation stage of the biofilm.
In the maturation stage, Golden staph undergoes relatively high cell division forming the extracellular matrix of the host factor and extracellular DNA (eDNA). Recent studies have revealed that the array also contains biomolecules such as polysaccharides, proteins and polysaccharide intercellular antigens (PIA) (Wallace, 2017) [8]. PIA is primarily composed of b-1, 6-linked N-acetylglucosamine residues but also contains anionic fraction and some traces of non- N-acetylated D-glucosaminyl. The compound is synthesized by the in vitro UDP-N-acetylglucosamine activities and gives the biofilm its toxic properties.
The structure has diversified protein components with coordinated gene expression. This diversity is vital for its survival mechanism against antibiotics. This biofilm then keeps on growing, sometimes forming mushroom- or tower-like structures that are maximally resistant to antibiotics or other antimicrobial agents. After a specific period, auto-inducing peptide (AIP or octapeptide) accumulates within the matrix and activates the histidine kinase. The histidine kinase then phosphorylates the response regulator and establishes P3 promoter transcription. The process releases RNA molecules which regulate the release of virulence factors causing the dispersal of the biofilm cells.
The bacterial cells can move to other areas and initiate the development of new biofilms. The biofilm’s bacteria clusters can be recognized under a light microscope. The pathogen’s precise identification can only be conducted through DNA hybridization and specialized staining techniques (Høiby et al.,). Benson, however, presents several incidences of PIA-independent biofilm developments including are gene locus coded activities, fibronectinbinding proteins, biofilm-associated protein (Bap) and Bap-related proteins. The processes of cell division and extracellular matrix deposition continue as the film accumulates into glycocalyx or slime layers. Golden staph cells within the matrix sometimes reactivate the planktonic state, causing the dispersal of the film (Bookenberger et al.,) (Figure 1).
Biofilm Antibiotic Resistance Mechanisms
Different antibiotics and antimicrobial agents have been tested
against S. aureus biofilms. However, these studies have consistently
shown that the antibiotic’s susceptibility has significantly reduced
in the biofilm mode of growth as compared to when they are grown
in planktonic mode [7,8]. The increased antibiotic resistance is
linked to their targeted mutations mechanism, decreased cell permeability to the antimicrobial agents and chemical modifications
of the enzymes.
Variable mutation across these biofilm layers and hyperactivity
at the bacterium are the two aspects that increase the matrix’s
resistance to antimicrobial agents. The bacteria have a higher rate
of mutation and transfer their genetic material horizontally, thus
becoming highly resistant to multiple antibiotics. The bacteria
cell can also alter the target so that its affinity for binding to the
antimicrobial is significantly reduced.
Effect of Flucloxacillin against Staphylococcus aureus
Flucloxacillinis a narrow-spectrum beta-lactam antibiotic that belongs to the class of antibiotics called penicillin. Historically, it has been shown to be effective against penicillinase-producing strains of the bacteria such as S. aureus. It is an isoxazole penicillin antimicrobial agent that is well absorbed after oral or intramuscular administration. As compared to other types of isoxazole penicillin’s, flucloxacillin has been found to be effective against Gram-positive cocci which are now inherently penicillin-resistant (Sutherland et al., 1970). The mechanism of action of flucloxacillin is the same as for other beta-lactams; it acts by inhibiting the cross linking of the linear peptidoglycan polymer, thus inhibiting synthesis of the bacterial cell wall. Because the cell wall in Gram positive bacteria such as S. aureus has a thick peptidoglycan, the integrity of the cell wall is compromised, and cell death occurs.
Problem Statement
S. aureus was first discovered in human abscesses and presented as a causative agent in wound infection in 1880 at Berlin Surgical Congress by a Scottish surgeon, Alexander Ogston[3]. From then on, the bacterium has continued to colonize the human population and emerged as one of the significant skins and soft tissue infectious pathogens. S.aureusbecame a significant clinical challenge and community health concern because it was linked to several cases of recurring, persistent and chronic infections including rhinosinusitis, osteomyelitis and periodontitis.
The bacterium rapidly evolved and developed different resistance mechanisms against the human immune system and antibacterial drugs. S. aureushas an immunomodulatory virulence mechanism and adhesion characteristics that alter the immune response activities of its host. Consequently, a series of clinical preventive and treatment techniques have been established. Such methods include carbolic acids (chlorhexadine and mupirocin), glycol peptides (teicoplanin vancomycin) and oxazolidinones (OXAs).
Current developments in the medical field include the introduction of solithromycin (SOL),fluoroquinolones,ozenoxacin, nemonoxacin and zabofloxacin. However, the efficacy of such technologies is limited by several factors and most importantly antibiotic agents are only effective in the early phase of infection because they cannot penetrate the biofilm matrix. Clinical protocols such as aminoglycoside nephrotoxicity and the surgical removal of the bacterium biofilm have been found to be complex and costly. Advanced antibacterial therapy is, therefore, an unmet requirement.
Resolution
Consequently, we introduce a novel antibacterial technology where Flucloxacillin and D-amino acids (D-glutamate, D-aspartate and D-methionine) are combined to develop a new S. aureus biofilm dispersal polytherapy. The minimum inhibitory concentration (MIC) and minimum lethal concentration (MLC) of flucloxacillin were determined and the inhibitory effect of each of the D-amino acids on biofilm was separately assessed. Subsequently, flucloxacillin was combined with the amino acid and its effectiveness was examined at the optimum amino acid concentration, comparing it to that of pure flucloxacillin. Statistical analysis was also undertaken to establish the clinical significance of the novel idea.
Objectives
The aim of the study is to investigate the antibacterial effectiveness of flucloxacillin when combined with D-amino acids (D-glutamate, D-aspartate and D-methionine) on S. aureus biofilm. The data are compared with the effectiveness of flucloxacillin alone. We hypothesise that D-Amino acids will improve the antibacterial efficacy of flucloxacillin when combined. The specific objectives include:
a) To examine minimum inhibitory concentration (MIC) and
minimum lethal concentration (MLC) of flucloxacillin.
b) To analyse three D-amino acids’ efficacy on the S. aureus
biofilm separately.
c) To explore the efficacy of flucloxacillin when combined
with each of the three D-amino acids at optimum concentration.
d) To compare the antibacterial effectiveness of the
combinatorial agents and flucloxacillin alone.
e) To undertake a statistical test (ANOVA) to validate the
clinical significance of the newly developed antibacterial agent
Materials and Methods
Health and Safety
All experiments were conducted using aseptic techniques and in accordance with the University of Wolverhampton’s code of practice regulations for project work in the School of Pharmacy. All safety precautions such as the wearing of lab coats, gloves and safety glasses were followed at all times, working in a class 2 microbiological safety cabinet (BioMAT 2) when handling Staphylococcus aureus, and appropriate storage and disposal of materials was followed. Also, COSHH risk assessments were carried out for all hazardous equipment used during the experimental work
Growth Media Preparation
Tryptone soy broth media (TSB) was prepared by dissolving the
sample media in water and autoclaving the solution for 15 minutes
at 121°C and 15Psi. 12g TSB with 30g/l initial concentration
was added to 400ml of distilled water. Part of the media was
then transferred to ten separate test tubes and autoclaved. The
remaining sample was autoclaved in the universal bottle to be used
in the overnight setup.
Similarly, 14.8g of Tryptone soy agar TSA was dissolved in the
equivalent volume of water (400ml) and autoclaved for 15 minutes
at 121°C and 15Psi. The agar was then poured into a Petri dish while
warm to prepare plates. The plates and media were stored safely
for the next laboratory session. It was of vital importance that both
reagents remained free from contamination throughout the whole
laboratory exercise.
Preparation of the Inoculum
Initially, a single colony of S. aureus was inoculated into 10 mL of LB broth in a sterile universal bottle and grown overnight at 37°C on a rotary shaker at 150 rpm. The culture was then adjusted to an optical density of 0.1 (1 × 107 CFU/mL) at 600 nm.
Broth Dilution Assay
A detailed description of the antimicrobial agents to be used for master and working stocks solutions preparation were as shown in Index 1. Minimum inhibitory concentration (MIC) and minimum lethal concentration (MLC) were analysed using a Broth dilution assay. The Broth dilution test is a laboratory analytical tool for examining bacterium’s susceptibility to antimicrobial drugs. Different antimicrobial concentrations incorporated into growth media are exposed to a standard number of bacterium cells and the microbe’s growth is assessed.
In the present study, serial antimicrobial agents with varying concentrations were prepared by a sequential double dilution of the antimicrobial agents provided using TSB prepared in the previous laboratory session. 5ml AgNO3 of the initial level was added to 5ml TSB to obtain ½ the dilution. Half the volume of the resulting solution (5ml) was transferred to 5ml TSB to achieve a dilution of ¼. This procedure was repeated until a dilution of 1/256 was achieved. The dilution series is as shown in Table 1. Tubes 9 and 10 were left undiluted for control tests. 100μl dilute inoculum was added to the first antimicrobial samples in the dilution series (Table 1). The samples were incubated for 18 hours at 37oC in a static incubator and growth was recorded.
Biofilm Formation
The biofilm formation was conducted in 96-well plates
according to the directions of Toole (2011). 100 μL aliquots of
the adjusted bacterial suspension were inoculated into individual
wells of the 96-well flat-bottomed polystyrene plate under aseptic
conditions overnight at 37°C for 72 hours in the static incubator.
The excess culture was discarded, and the plate gently washed
using 100μl of 1X phosphate buffered saline (PBS; pH 7.4) using a
multichannel pipette.
The regulations guiding toxic sample disposal were observed.
The wells were stained using 0.1% Crystal Violet for 30 minutes
at room temperature. Excess crystal samples were washed out
four times using distilled water and dried using a paper towel. The
plate was then set upside down to dry overnight and a snapshot
captured the appearance of the dry wells coated by the biofilm.
The 96-well plates were incubated for 15 minutes in preparation
for biofilm quantification. 125μL solubilize biofilm (95% ethanol)
was added to the wells and the samples were incubated for another
15 minutes. The125μl solubilized CV strains were transferred in a
flat-bottomed microtiter dish and OD at 590nm was recorded. 95%
ethanol was used as the blank. The biofilm formation assays were
performed in triplicate and the absorbance recorded.
Biofilm Dispersal Assay Using D-amino Acid Solutions
Initially, 2.13g, 2.35g and 5.66g of D-aspartic acid, D-glutamic
acid and D-Methionine acid respectively were dissolved in sterilised
distilled water to obtain 100mL with a concentration of 160mm.
The samples were left to stir on a magnetic stirrer for 30 minutes
until the amino acid was fully dissolved. After that, amino acids
were filter sterilized. The amino acid stock samples were stored in
aliquots at a temperature below freezing point (-30°C).
The solutions were diluted to provide two-fold the recommended
concentration by the protocol. Double strength MHB was then
prepared, autoclaved and added to the D-amino acid samples to
obtain the serial of two-fold dilution of the D-amino acid/ MHB
combination. 80mM, 40mM, 20mM, 10mM and 5mM were added to
the 96-well plate containing the bacteria biofilm. The experiment was then incubated for 72 hours at 37°C and the biofilm bacterium
inhibition effect investigated. The biofilm dispersal protocol was
conducted in triplicate for all three of the D-amino acids tested and
the average effect compared to the previous lab exercise.
Biofilm Dispersal Using D-Amino Acids/ Flucloxacillin Combinatorial
Each of the D-Amino acids was prepared to the optimum concentration marked as the most efficacious dilution in the previous exercise. Four times the MLC (5mg/ml) Flucloxacillin was added to the D-Amino acid solution. Three other samples were prepared and used for comparison; typically, 5mg/ml Flucloxacillin, pure MHB and D-Amino acid at its optimum inhibition concentration. 100uL of 1 × 107 cells/mL inoculum was added to the 96-well plate containing the bacteria biofilm. The experiment was then incubated for 24 hours at 37°C and the biofilm bacterium inhibition effect investigated. The biofilm dispersal protocol was conducted in triplicate for all four sample acids tested and the averaged result compared to the D-amino acid and Flucloxacillin separate tests. Antibacterial efficacy was investigated based on the spectrophotometer concentrations readings.
Statistical Analysis
In order to determine the clinical significance, an analysis of variance (ANOVA) test was conducted on the data that demonstrated potential antibacterial efficacy.
Results
Broth Dilution Assay
The values of flucloxacillin’s MLC and MIC against S. aureus were 1.25 mg/ml and 0.625 mg/mL respectively (Figure 2.1). The minimum concentration was observed in the test tube with the respective concentration level. Bacteria growth was observed to be increasing in concentration from test tubes 4 to 9. Test tubes 1, 2, 3 and 10 were clear (Table 2.1). The minimum inhibitory level was taken as the lowest concentration with no bacteria growth observed [T4 = 0.625]. Test tube 10 was used as a negative control test. Similarly, an MLC test is also presented below (Table 2.2).
Figure 2.1: Broth dilution test for flucloxacillin MIC. The lowest concentration with no bacteria growth was recorded in test tube 4.

Effect of D-Amino Acids
The study investigated the impact of adding amino acids to the antimicrobial Staphylococcus aureus biofilm growth. Three D-amino acids (D-Aspartic acid, D-Methionine acid and D-Glutamic acid) were first tested separately. The assessment of the antibacterial efficacy of the amino acids was made by taking series of optical absorbance of each biofilm after they had been exposed to varying concentrations of the samples (Figure 2.2). The effectiveness of the tested samples was analyzed from their ability to reduce the bacteria concentration in the biofilm.
D- Methionine and D-Aspartic acid indicated uniform antibacterial effectiveness on the biofilm. The concentration of the bacteria was observed to drop with the concentration of the antibacterial agents (Figures 2.3 & 2.4) (Tables 2.3 &2.4). The trend continued until an optimum level was reached above which the bacteria concentration was observed to rise with the increase in antibacterial agent concentration. Both D-Amino acid samples reach their optimum efficacious concentration at 20mM
However, D-Methionine acid showed similar effectiveness at 40mM, contrary to D-Aspartic whose activity started to decline with a rise in acid concentration above its optimum value. Again, a significant drop in antibacterial activity at 10mM was observed in both samples against the trend. This discrepancy was attributed to experimental errors including sample contamination, measurement error and non-uniform incubation conditions. Analysis of variance, however, showed that only D-Methionine was of clinical importance (P-value = 0.161>0.05). D-Aspartic has less P-value and would be rejected for clinical application.
On the other hand, the D-Glutamic acid test showed that sample concentration increased the rate of bacteria growth (Figure 2.5). A uniform rise in S. aureus concentration in the biofilm samples was observed with an increase in the D-Amino acid except for 40mM where a significant drop was evident. The observation in the three D-Amino acid samples used is summarized as shown in Figure 2.6.
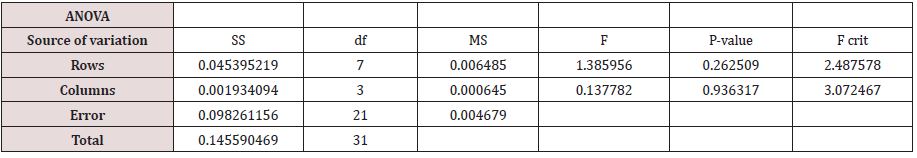
The antibacterial activity of the three D-amino acid samples was also investigated when combined with flucloxacillin (Figure 2.7). Only D-Methionine showed an improved rate of antibacterial activities when used in combination with flucloxacillin in comparison to the pure flucloxacillin and amino acid samples used separately. The sample recorded OD595 of 0.057 compared with MLC of 0.009 and Methionine of 0.153. Additionally, the ANOVA test indicated that the sample was of clinical significance as compared to its pure amino acid test Figure 2.8 & Table 2.5.
Figure 2.7: Flucloxacillin/D-Amino acid antibacterial test; a) D-Aspartic; b) D-Glutamate; and c)D- Methionine

On the other hand, the other two antibacterial agents prepared showed an increasing effect on bacteria growth when combined with flucloxacillin instead. Higher concentrations of the bacteria in the biofilm were observed when the inoculum was subjected to the combinatorial culture as compared to the two cases where the samples were used separately. The mixture recorded an OD595 of 0.89, compared to pure D-Amino acid of 0.49 and MLC of 0.18 in D-Aspartic acid (Figure 2.9). Similarly, D-Glutamate recorded OD595 of 0.89 when combined with flucloxacillin, which was relatively higher compared to MCL (0.19 OD595) and the pure sample (0.49 OD595; Figure 2.10).
In summary, only two D-Amino groups (D- Methionine and D-Aspartic acid) out of the tested samples showed the inhibition effect when used separately. However, only D-Methionine indicated significant consistency in the ANOVA test. The other sample showed an increased bacterial growth rate. When used in combination with flucloxacillin, only D-Methionine was effective. ANOVA indicated that the test would be consistent and, therefore, is of clinical significance. The MLC and MIC values of flucloxacillin against S. aureus were 1.25 mg/ml and 0.625mg/mL respectively.
Discussion
In the given study, an analysis was undertaken to investigate
the efficacy of pure flucloxacillin and in combination with D-amino
acids (D-glutamate, D-aspartate and D-methionine) against S.
aureus. Although the mechanism of D-amino acids against biofilm
remains a mystery, studies have indicated that these compounds
have a significant effect on the matrix complex [12,13]. Von Dach
[12], for instance, determined that the bacterial signalling agents
responsible for inducing biofilm dispersal are functionally analogous
to D-amino acids. Huppert (2015) isolated sample D-Amino acids
from Bacillus subtilis biofilms and concluded that the substance
was a biofilm synthesis inhibitor. According to Marking & Shaw
[13], D-Amino acid molecules disrupt extracellular bonding and
disassemble the structure. As such, they offer a potential clinical
medication.
From the experiment, only D-Methionine and D-Aspartic acid
demonstrated a significant antibacterial effect on the biofilm when
the amino acids were used separately. D-Aspartic acid, however,
showed non-significant P-value in the ANOVA test, indicating that
the agent may not be of clinical value. The D-Glutamic acid test, on
the other hand, indicated an increase in bacteria growth with the
concentration of the antimicrobial level. Flucloxacillin recorded
1.25 mg/ml MLC and 0.625 mg/mL MIC against the bacterium [14].
Only D-Methionine demonstrated significant growth inhibitory
activity against S. aureus biofilm when used in combination with
flucloxacillin. In the remaining two samples, a higher concentration
was recorded for the mixture as compared to when constituents were
used separately. From ANOVA, the D-Methionine test demonstrated
a significant consistency, indicating that the compound offers a
promising potential clinical antibacterial therapy [15].
The results in this study are comparable to several other
studies in the field (Dahners; Wallace, 2017). Wallace [7] for
example, identified D-Amino acids as the components of biofilm
peptidoglycan. According to the study, the amino acids form peptide
chains and crosslinks with N-acetylmuramic acid and develop a
three dimension-like layer that gives the bacterium’s biofilm its
mechanical integrity. Additionally, the study identified that the
structure was relatively thick in Gram-positive bacteria and forms
80% of the biofilm’s dry weight.
In reference to Woehl [7], penicillin binds to DD-transpeptidases,
thereby altering oligopeptide crosslinks of peptidoglycan. The
deformation not only compromises the mechanical integrity and
allows accessibility of immune response agents but also inhibits
gene transfer across the biofilm. S. aureus, therefore, is incapable
of evolving to develop antibacterial resistant genes. It was also
shown that N-acetylmuramide glycanhydrolase which is a protein
in nature hydrolyses glyosidic bonds in the peptidoglycan matrix.
This therefore indicates that amino acids play a significant role in
building and breaking the biofilm matrix [16-18].
The therapeutic target of the structural protein component
within the matrix can be a potential clinical tool against biofilm
dispersal. More importantly, D-amino acid therapy can prevent
biofilm development and make the bacterium susceptible to
other antimicrobial and body immune response agents. The
concept, therefore, explains the improved performance observed
with D-Methionine acid on flucloxacillin antibacterial activity.
Additionally, flucloxacillin is reported to bind with penicillinresistant
N-acetylmuramide glycanhydrolase [19-21].
Dahners (2015) also presents D-Amino acids as an officious
Pseudomonas aeruginosa biofilm dispersant. The study
investigated the effect of adding sample D-Amino acids to three
antibacterial agents; typically penicillin, chlorhexidine and nisin
and showed that D-Trp and D-Met have significant antibacterial
influence against Pseudomonas aeruginosa biofilm. Dahners
(2015) agrees with Wallace (2017) that the D-Amino acids are
structural components of peptidoglycan and play a significant role
in regulating the formation and breakdown of biofilm components.
Notably, Dahners (2015) postulated that amino compounds alter
the exopolysaccharide components of peptidoglycan, thereby
reducing their mechanical integrity and decomposing the biofilm
matrix.
Damian (2017) further adds that amino compounds
demonstrated a significant inhibitory effect against biofilm
formation. The process of biofilm formation involves selective
protein uptake. The structural function of the protein blocks in
the biofilm matrix could be associated with the trend observed
with D-Glutamate. The sample facilitated bacteria multiplication,
implying that the D-Amino acid was a prerequisite material
for biofilm development. Only very few studies, however, have
examined amino acid consumption during biofilm development.
We thus propose the role of am
Conclusion
The current study has established a novel antibacterial therapeutic therapy. Three groups of D-Amino acids (D-glutamate, D-aspartate and D-methionine) were investigated with regards to their role in S. aureus biofilm dispersal activity. The samples were then tested in combination with flucloxacillin. The minimum lethal concentration of flucloxacillin was 1.25mg/mL, while the minimum inhibitory concentration was 0.625mg/mL. D-aspartate and D-methionine showed a significant antibacterial effect when used separately. However, there was an inconsistency in D-aspartate, thereby nullifying for clinical treatment (0.016<0.05). D-Glutamate was observed to facilitate the growth rate. On the other hand, only D-methionine was observed to improve the antibacterial activities of flucloxacillin. The ANOVA indicated that the D-Methionine test had a significant consistency and that the technique offers a promising potential clinical antibacterial therapy.
References
- Westhoff P (2015) Metabolic Aspects of Host Pathogen Interactions Revealed By Metabolomics.
- Huppert LA (2015) Characterization of The Bacillus Subtilis ESX-Type Protein Secretion System.
- Wallace IR (2017) The Importance ofStaphylococcal Superantigen-Like Proteins (Ssls) In Staphylococcus Aureus Pathogenicity, With A Focus On Ssl11.
- Todar K (2008) Staphylococcus aureus and Staphylococcal Madison, Wisconsin, USA.
- Gardete S, Tomasz A, (2014) Mechanisms of vancomycin resistance in Staphylococcus aureus. The Journal of clinical investigation 124(7): 2836-2840.
- Foster T (1996) In: Baron S, editor. Medical Microbiology. (4thedn). Galveston (TX): University of Texas Medical Branch at Galveston Chapter 12.
- Woehl JL (2017) Structure/Function Analysis of The Staphylococcus Aureus Extracellular Adherence Protein and The Human Innate Immune System. Manhattan, Kan, Kansas State University.
- Weidenmaier C, Lee JC (2017) Structure And Function Of Surface Polysaccharides Of Staphylococcus Aureus. Curr Top Microbiol Immunol 409: 57-93
- Van Der Horst AS, Medda S, Ledbetter E, Liu A, Wein hold P, et al. (2015) Combined Local and Systemic Antibiotic Treatment Is Effective Against Experimental Staphylococcus Aureus Peri-Implant Biofilm Infection. Journal of Orthopaedic Research 33(9): 1320-1326.
- Dryden M, Andrasevic AT, Bassetti M, Bouza E, Chastre J, et al. (2010) A European Survey of Antibiotic Management of Methicillin-Resistant Staphylococcus AureusInfection: Current Clinical Opinion and Practice. Clinical Microbiology and Infection. 16: 3-30.
- Chen D, Qian X, Hu Y (2017) Brief History of Bacteria, A. Singapore, Chemical Industry Press.
- Von Dach E, Harbarth S, Guillemot D, Durand Zaleski I, Bertrand X (2017) Methicillin-Resistant Staphylococcus aureus (MRSA): Prevention and Fight Against This Pathogen. Thèse De Doctorat: SantéPublique - Recherche Clinique: Paris Saclay.
- Marking DN, Shaw LN (2015) Exploring the Role of Intracellular Aminopeptidases in Staphylococcus Aureus
- Benson HJ (2015)Benson's Microbiological Applications: Laboratory Manual in General Microbiology.
- Bookenberger L,Hoet A (2017) Presence, Distribution, And Risk Factors Associated with Staphylococcus Aureus Among Veterinary Health Care Workers at The Ohio State University Veterinary Medical Center.
- Damian A (2017) Role of The Aryl Hydrocarbon Receptor in Disease Tolerance to Staphylococcus Aureus.
- Donlan RM, (2002) Biofilms: Microbial life on surfaces. Emerging infectious diseases 8(9): 881-890.
- Edmiston CE, Mcbain AJ, Roberts C, Leaper D (2015) Clinical and Microbiological Aspects of Biofilm-Associated Surgical Site Infections. Adv Exp Med Biol 830: 47-67.
- GarciaLara, Jorge, Weihs, Felix, Ma, Xing, Walker, etal. (2015). Supramolecular Structure in The Membrane Of Staphylococcus Aureus. National Academy of Science.
- Hood IV (2015) Structural and Biochemical Studies of Replicative Helicase Loading in Bacteria. Thesis University of California, Berkeley, USA.
- Jansen Van Vuuren,SJ (2015) Identification of Methicillin-Resistant Staphylococcus Aureusin Horses Using Conventional and Molecular Techniques.
- Penders J, Stolzoff M, Hickey DJ, Andersson M, Webster TJ (2017) Shape-Dependent Antibacterial Effects of Non-Cytotoxic Gold Nanoparticles. Dove Press 12: 2457-2468.
- Tang Y, Ali Z, Zou J, Jin G, Zhu J, et al. (2017) Detection Methods for Pseudomonas Aeruginosa: History and Future Perspective. Rsc Advances.
- Tran HL (2017) Saturation Mutagenesis and Structure-Activity Relationship of a Natural Product Antibiotic. Dissertation Abstracts International 78-09.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...