
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4579
Research Article(ISSN: 2637-4579) 
Preparation of Carbon Nanotube Reinforced Gelatin-Chitosan-Hydroxyapatite Biocomposite for Bone Tissue Engineering Volume 1 - Issue 3
Tanjina Islam1, Khandaker S Salem1, Shanta Biswas1, Papia Haque1, Sunzida H Rimu2 and Mohammed Mizanur Rahman1,2*
- 1Department of Applied Chemistry and Chemical Engineering, University of Dhaka, Bangladesh
- 2National Institute of Textile Engineering and Research, Bangladesh
Received: February 23, 2018; Published: February 28, 2018
Corresponding author: MM Rahman, Department of Applied Chemistry and Chemical Engineering, University of Dhaka, Bangladesh, Tel: +880-2-9661920-70/7392, Fax: +880-2-9667222; Email: mizanur.rahman@du.ac.bd
DOI: 10.32474/OAJBEB.2018.01.000113
Abstract
Uniform and highly stable dispersion of multi wall carbon nanotube (MWCNT) in aqueous solution using surfactant sodium dodecyl sulphate was prepared by physical adsorption of surfactant molecules on to the nanotubes which overcomes the van der Waals forces and prevents aggregation between individual nanotubes. Chemically functionalized MWCNT dispersion was also prepared by mixed acids using (3:1) (H2SO4: HNO3) that was obtained to be less cytotoxic than the physically functionalized MWCNT dispersion. Chemically functionalized MWCNT-Hydroxyapatite (HAp)-Chitosan-Gelatin composite scaffold samples were prepared by solution casting method after optimizing the cytotoxic effect results. The effect of varying content of MWCNTs on the physico-mechanical, thermal, morphological properties of the nanocomposites was evaluated. The chemical change of composite with varied MWCNT content was studied using FTIR and morphological characterization was done by SEM, where Porous structure was observed on the composites, which is supposed to be required as criteria of a bone scaffold to grow bone tissues. Compressive Strength (CS) of composite scaffold increased by 95.82% with increase of MWCNT content (from 0.1% to 0.25%). The thermal characterization of nano-composites was done by TGA and DSC and it was found that the nanocomposite containing 0.25% MWCNTs showed highest thermal stability. TGA of nanocomposites containing 0.25% MWCNT increased by 363.16% in comparison to that of nanocomposites containing 0.1% MWCNTs. However, due to highest cytotoxicity of nanocomposite having 0.25% CNTs, 0.1% composition was considered the best.
Introduction
Over the last four decades, there is a growing interest in the field of artificial organ material preparation, transplantation, surgical reconstruction and the use of artificial prostheses to treat the loss or failure of an organ or tissue [1]. Autograft and allograft are considered ultimate for bone grafting procedure providing osteoconductive and osteoinductive growth factors. However, limitations in donor site, additional surgery, disease transmission and expenditure poses a need to develop alternatives to autograft and allograft [2]. The repair and replacement of injured or defect bone is a critical problem in orthopedic treatment throughout worldwide. In recent years, significant development has been made in organ replacement, surgical reconstruction and the use of artificial prostheses to treat the loss or failure of an organ or tissue [3]. Due to limited supply of natural bone for grafting, the need for synthetic bone substitutes which possess same physiochemical and biological properties as natural bone is ever increasing. These limitations and concerns created substantial interest in the development of artificial materials as bone graft substitutes or exenterate. At present days, cell and scaffold based tissue engineering treatment are being explored for a better treatment of bone related ailments [4].
Several techniques and materials have been employed for the orthopedic treatment for the past several decades. Bone is mainly composed of organic and inorganic portion such as collagen and nano hydroxyapatite. The prepared composite materials should mimic the natural function of bone when it is replaced; moreover, it should replace the function of extracellular matrix and should not be toxic to surrounding tissue environment, allowing cells to proliferate and differentiate in a normal pace. In bone tissue engineering, biodegradable and biocompatible materials introduced at the defective region should mimic all the natural functions of the normal bone such as porosity, cell proliferation, etc. In the recent years, natural polymers are being considered by researchers for tissue engineering due to their biocompatibility and biodegradability nature. The well-known limitations associated with clinical use of autografts and allografts continue to drive efforts to develop bone graft substitutes using the principles of biomaterials and tissue engineering. Artificial bone substitutes usually include metals, ceramics (e.g., hydroxyapatite (HAp) and/ or MWCNT, Chitosan etc.) [5,6]. Artificial bone substitutes are effective in bone defect repair; however, they are not ideal. Metallic implants may cause atrophy of surrounding tissue through stress shielding, requiring corrective procedures. Ceramics can aid in the healing of the bone defect by acting, preferably temporarily, as a scaffold for bone in-growth [7]. Almost all of the materials have some drawbacks and their selection usually requires some degree of compromise. Thus, the search for novel biomaterials as alternatives for pure biomaterials remains an important topic in medical research. Because of the possibility of combining the advantages of different pure materials, composite biomaterials are more attractive than pure biomaterials. Recently, many composite materials have been developed for biomedical applications and they include ceramics/ceramics composites (for example, HA/plaster of Paris, calcium phosphate/calcium carbonate), polymer/polymer composites (PLA/collagen), ceramic/metal composite (HA-coated Ti), and ceramics/polymer composites (HA/CNT, HA/collagen and HA/chitin) [8,9]. Kim et al. [10] developed a biodegradable composite scaffold using chitosan grafted with functionalized multiwalled carbon nanotube in addition to HA (f-MWCNT-g- chitosan/HAp) scaffolds were prepared for the first time via freeze- drying method and physiochemically characterized as bone graft substitutes. The major objective of this research work is to develop indigenous technology for substituting the traditionally used metal bone fracture fixation devices and to develop biodegradable, biocompatible scaffolds for bone tissue engineering using gelatin, chitosan, hydroxyapatite, MWCNT. These composite scaffolds from biopolymer and bioactive ceramic materials could provide an optimum environment for cell growth, and then organized into a real bone.
Material and Methods
Materials
MWCNTs with average diameter of 9.5nm and average length of 1.5|im were purchased from NANOCYL (NANOCYL™ NC7000 series, Germany). These nanotubes were synthesized by chemical carbon vapor deposition (CCVD) method. The carbon purity, metal oxide, and surface area of these thin p-MWCNTs were 90 %, 10 %, and 250-300m2/g, respectively. Chitosan was prepared from waste prawn shell as described in our previous investigation [11]. The viscosity average molecular weight and DD of extracted chitosan were 167,231 Da and 84.4 %, respectively. Sodium hydroxide (Merck, Germany; purity >97 %), fuming hydrochloric acid (Merck, Germany; 37 % solution), glacial acetic acid (BDH Chemicals Limited, England), nitric acid (Active Fine Chemicals, Bangladesh, 65 %), acetone (Active Fine Chemicals, Bangladesh), and ammonia solution (Merck, Germany) were used without further purification.
Methods
Extraction of hydroxyapatite from waste eggshell
Hydroxyapatite nanocrystals have been prepared according to the procedure describe in the literature [12]. In a typical procedure, chicken egg shells were firstly cleaned in boiling water and calcined in air using an electric furnace at 900°C (heating rate of 10°C min- 1) for 1h. The calcined powder was ground in an alumina mortar- pestle. This CaO powder was completely dissolved in concentrated HNO3 and was diluted with distilled water such that 1M calcium nitrate (Ca (NO3)2 CN) was obtained. 0.6M potassium dihydrogen phosphate (KH2PO4) solution in distill water. This solution mixture was kept constant at pH=10 by adding ammonia. The solution was rigorously stirred for 1h and kept for ageing overnight at room temperature. A bilayer solution of clear ammonia at the top and white precipitate powder at the bottom of the beaker was obtained. The excess ammonia solution at the top was poured out and the precipitate was washed with distilled water to remove the presence of NH4+ and NO3- ions. This precipitate was filtered out using filter paper and allowed to dry at 650C for 24 h in a dry oven. The resultant cake obtained was calcined in air at different temperatures ranging from 150 to 7000C for 30min using an electrical furnace and employing a heating rate of 100C/min. The Fourier transform infrared spectroscopy (FT-IR) was done with Nexus 6700 FT-IR (Thermo-Nicolet, Inc.) [13]
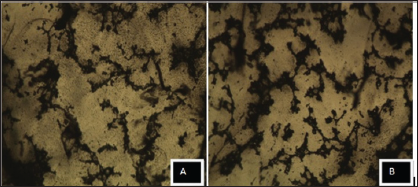
Figure 1: Cytotoxic analysis of sample A and B are 0.1 and 0.2% MWCNT dispersions respectively in chloroform.
Preparation of MWCNT dispersion
50mg of MWCNTs was added into100 ml distilled water containing 1% surfactant (SDS- Sodium Dodecyl Sulphate). Ultrasonication of the solution containing MWCNTs was carried out for 40 hours in 50-60HZ at 200W bath ultrasonicator. Then the sonicated solution was centrifuged at 10,000rpm in high speed refrigerated centrifuge H-9R KOKUSAN CORPORATION, JAPAN, for lhour followed by filtration using vacuum filter (pore size 0.45|im) of the solution to remove the non-dispersed agglomerated MWCNTs. A well dispersed MWCNTs solution of homogeneous size range was obtained as shown in (Figures 1-4).
Figure 2: FT-IR spectra of composite samples ((a) sample A, (b) sample B, (c) sample C, (d) Gelatin-Chitosan (97.5:2.5), (e)Gelatin- Chitosan-Hydroxyapatite (87.5:2.5:10).
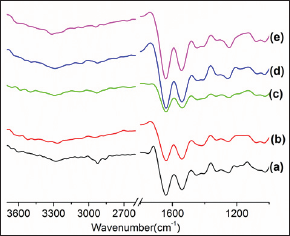
Figure 3: SEM images of a) gelatin-chitosan composite, b) gelatin-chitosan-hydroxyapatite, c) sample A and d) sample C.
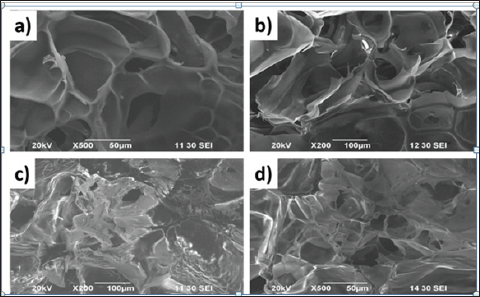
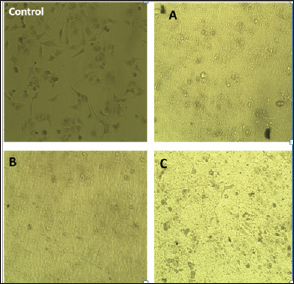
Figure 4: Cytotoxicity analysis of composite A, B and C.
Primary functionalization of MWCNT
MWCNTs were functionalized primarily by treating them with mixure of concentrated H2SO4/HNO3 (3:1) (v/v). HNO3 of 60% strength and H2SO4 of 98% strength were used for this purpose. 175ml of mixed acid was taken in a stopper bottle and 350mg of MWCNTs was added. The treatment was carried out with sonication at 40°C for 6hours. After acid treatment, MWCNTs were filtered and then washed using deionized water until the pH value was reached to 7. The neutralized MWCNTs were then dried at 80°C for 24h to get dry powder of functionalized MWCNTs .The resulting material is termed as primary functionalized MWCNTs (CNT-COOH MWCNTs) [14-16].
Preparation of the nanocomposite
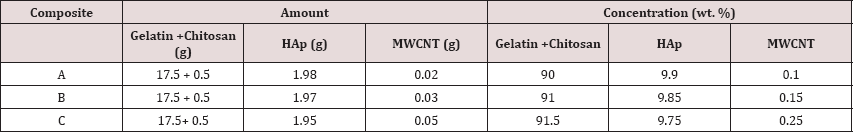
As MWCNT dispersion of (0.1% to >0.3%) showed noncytotoxic effects, three different samples were prepared of three different compositions of CNT. As shown in (Table 1).
Table 1: Composition of different components in the nanocomposite.
Characterization of composites
FT-IR analysis
The dried sample was embedded in KBR pellets analyzed by an ATR-FTIR (Attenuated Total Reflectance/ Fourier Transforms Infrared) spectrophotometer (Model-01831, SHIMADZU Corp, Japan) .The composites were analyzed by ATR-FTIR. The spectra were recorded in both cases in the absorption band mode in the range of 4000-400 cm-1.
Surface morphology
A Scanning Electron Microscopy (SEM) was used to determine surface morphology of the composites. SEM analysis has been carried out using a JEOL JSM-6490LA machine. The JSM-6490LA is a high performance scanning electron microscope with an embedded energy dispersive X-ray analyser (EDS) which allows for seamless observation. As the surface morphology of the samples were needed to be analyzed, the non-conducting samples were set on a carbon tape, made conducting by platinum coating and then placed for analysis at 20kV.
Thermogravimetric analysis
Thermogravimetric analysis of the composite samples was done using TG/DTA EXTAR 6000 STATION, Seiko Instrument Inc. Japan. The TG/DTA module uses a horizontal system balance mechanism. TGA measuring range: ±200mg (0.2|ig), DTA measuring range: ±1000|iV (0.06|iV), at < 1000m/min. Thermo gravimetric analysis (TGA) were performed of each sample using a Perkin-Elmer SetUp (TAQ-500) and a heating rate of 200C/min under a nitrogen atmosphere.
Differential scanning calorimetry (DSC) analysis
DSC tests were carried out using a DSC-60 (SHIMADZU Corp.) [(ASTM standards 2008a; ASTM standards 2008b, flow rate 20mL/ min, temp rate 10°C min-1, Aluminum cell]. The sealed Aluminium pan was put on the calorimeter along with an empty sealed pan. Change of heat per gram of sample was recorded at a constant temperature for 60minutes with a computerized system in dry nitrogen environment.
Compressive strength analysis
Compressive strength is the strength of materials, the capacity of a material or structure to withstand loads tending reduce size. It can be measured by plotting applied force against deformantion in a testing machine. some materials fracture at their compressive strength limit, others deform irreversibly, so a given amount deformation may be considered as limit for compressive load. Compressive strength was measured with Universal Testing Machine (Hounsfield, Model H50 Ks 0404, and UK).
Cytotoxicity analysis
Cytotoxic analysis was examined in Centre of Advanced Research in Sciences. In brief, HeLa, a human cervical carcinoma cell line was maintained in DMEM (Dulbecco's Modified Eagle's medium) containing 1% penicillin-streptomycin (1:1) and 0.2% gentamycin and 10% fetal bovin serum (FBS). Cells (4x10A4 /400|il) were seeded onto 24-well plates and incubated at 370C+5% CO2. Next day 100|il of sample (autoclaved previously) was added at each well. Cytotoxicity was examined under an inverted light microscope after 24h of incubation. Duplication wells were used for each sample. The measurement was carried out in autoclave (DAC-45, Human lab, korea), Biological Bio safety Cabinet (model: NU-400E, Nuaire, USA), CO2 Incubator (Nuaire, USA), Trinocular microscope with camera (Olympus, Japan).
Results and Discussion
Cytotoxic analysis of MWCNT dispersion
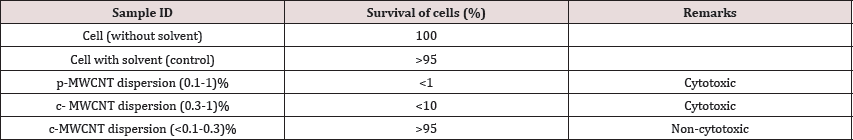
The physically functionalized MWCNT dispersion (1%) was prepared by 40h sonication with surfactant Sodium Dodecyl Sulphate (SDS) from pristine MWCNT. Chemically functionalized MWCNT (1%) was prepared by treating the pristine MWCNT with acid-mixtures of (H2SO4& HNO3) of (3:1) ratio. Physically (p) and chemically (c) functionalized dispersions (p-MWCNT and c-MWCNT) were diluted to varied compositions from 0.1% to 1%. Different samples of varied compositions were tested for cytotoxic analysis. From the analysis, we have observed that chemically Table 2: Cytotoxic analysis of physically and chemically functior functionalized dispersion yielded less toxicity than physically functionalized MWCNT dispersion. It also revealed that the increase of MWCNT (ranging from 0.1-1%), cytotoxicity is visibly increased. From this study it was also found that 0.1% chemically functionalized dispersion showed least cytotoxicity and survival of cells was higher than 95%. The overall cytotoxicity analysis data are presented in (Table 2):
Table 2: Cytotoxic analysis of physically and chemically functionalized MWCNT dispersion.
Characterization of composite
FT-IR analysis
From FT-IR analysis it was observed visible change in frequencies for N-H stretching of the composites as observed in (Table 3) which is probably due to the interaction with the key components of the composite and chitosan, where as we have seen that there is a characteristic band in peak of 3340cm-1 for chitosan. After incorporation of MWCNT in gelatin-chitosan and gelatin- chitosan-hydroxyapatite composites, the peaks in the region between 1306 to 1289cm-1 and 1399 to 1380cm-1 disappeared.
Table 3: Change in N-H stretching in the composites.
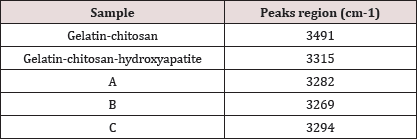
It is also clear from the figure that the addition of pristine nanotube does not causes any new bond because the bonding frequencies related to -OH, -C-O or -NH of the pure gelatin which have not been changed. The intensity of the bonds decreased due to presence of MWCNTs in the matrix. After incorporation of MWCNT in gelatin-chitosan and gelatin-chitosan hydroxyapatite composites, the peaks in the region between 1306 to 1289cm-1 and 1399 to 1380cm-1 disappeared.
Scanning electron microscope analysis
SEM analysis of the composites showed inters connective porous structure as shown in (Figure 4). At the low MWCNTs concentrations, the nano-composite surface is mostly smooth with little distinguishable MWCNTs visible at high resolution and the cross section) confirms that MWCNTs within the gelatin- chitosan- hydroxyapatite matrix. The porous structures and morphology of the composites give a good scope of application as bone scaffolds, as when used as bone scaffold, porous structure is required to grow bone tissue within the scaffold.
Cytotoxicity analysis of composites
The results of cytotoxicity analysis are shown in (Figure 5) and the data are presented in (Table 4). It was observed that with increase of content of MWCNT, cytotoxicity is increased. Survival of cells in the composite samples were >90 to 80% in the sample A and sample B.
Table 4: Cytotoxicity results of the biocomposites
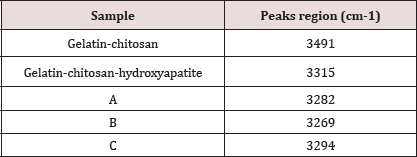
Figure 5: TGA analysis of composite samples ( (a) Gelatin-Chitosan, (b) Gelatin-Chitosan-hydroxyapatite (c) sample A, (d) sample B and (e) sample C.
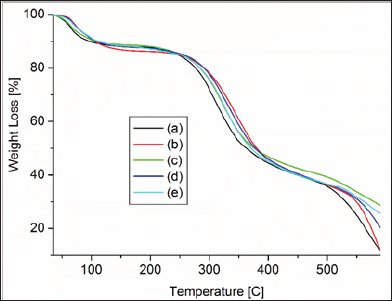
Thermogravimetric analysis
Figure 6: DSC analysis of composite samples ((a) sample A, (b) sample B, (c) sample C, (d) Gelatin-Chitosan-Hydroxyapatite and (e) Gelatin-Chitosan.
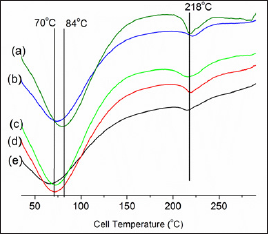
Thermogravimetric analysis (TGA) of the biocomposites are measured in given in (Figure 6). The TGA thermogram showed that MWCNTs-gelatin-chitosan-HAp nano-composites were thermally more stable than gelatin-chitosan and Gelatin-Chitosan- Hydroxyapatite composites. The reason of thermal stability of nano-composite may be due to physical-chemical interaction by carbon nanotube and biopolymer molecules. Another reason of thermal stability of nano-composite may be due to physical- chemical adsorption of gelatin-chitosan polymer chains onto the MWCNTs surface. MWCNTs can easily arrange themselves within the helical structure of gelatin-chitosan protein polymer chain due to their nano-size and make strong hydrophobic attraction with hydrophobic ends of amino acids chain. With increase of MWCNT in the composites thermal stability was observed to be increased. Residue in gelatin-chitosan composite is 5.89%, which is least and the residue for C is the highest with a value of 27.28%.
The DSC thermo grams of all of the composite samples without or with MWCNTs are presented in Figure 6. The figure showed one single broad glass transition peak (endothermic peak) with the maximum temperature within the range 600C-900C. However, the positions of Tg in the blended systems are found to be intermediate between those ofthe two pure polymers, indicating the compatibility of the blended systems. In addition, the transition widths of Tg for the blends are almost identical to those of the pure components, which further supports single-phase behavior of the blending system. The endothermic phase transition peak at 600C-900C may have been related to the helix-coil transition, which overlapped with the glass-transition temperature (Tg),where as the exothermic peak at 218°C was more uncertain, and it was probably associated with the denaturation of the gelatin segments. The broadness of the first peak is due to overlapping of Tg of the 18 amino acids present in the gelatin. The DSC curves of the MWCNTs-nano-composites shift toward higher temperatures compared with which might be attributed to the presence of MWCNTs that adsorbed the polymer as well as attached with strong van der Waal interaction and thus thermally stabilized the nano-composites.
Compressive strength
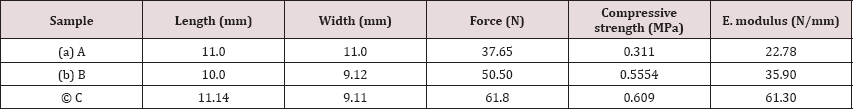
Compressive strength of the composites is seen to be increased with the increase of content of MWCNT (from 0.1% to 0.25%). Compressive strength is referred as force per area therefore, the force for sample A is 37.65 N, the compressive strength is 37.65N/ (10x10)mm2=0.311 N/mm2 or 0.311MPa and for the sample C, where the MWCNT is highest here, that is 0.25%, the compressive strength is 0.609MPa. The compressive strength data are given in (Table 5).
Table 5: Compressive Strength comparison of the composites.
Conclusion
To prepare a bionanocomposite for bone tissue engineering as an artificial bone scaffold, we have successfully incorporated MWCNTs in gelatin-chitosan-hydroxyapatite composite and found that the mechanical and thermal performances ofMWCNTs- gelatin-chitosan-hydroxyapatite nano-composites were satisfactory. Addition of functionalized CNTs helps boosting the conducting efficiency nano-composites. The experimental results of this research were confirmed through different analytical tests. Therefore, total elemental modeling will be part of the future work.The study paves the way for the development of a nano-composite for biomedical application especially in case of bone replacement aspects, since MWCNT is non-toxic within certain range of content in the composite. Increasing the loading of MWCNTs to gelatin-chitosan-hydroxyapatite nano-composites increases the morphological, physico-mechanical, thermal properties of the nano-composites. Compressive strength of the samples of having 0.1%, 0.15%, 0.25% MWCNT are 0.311, 0.554, 0.609 MPa respectively, which shows a considerable increase in mechanical property. Analyzing the same samples with thermogravimetric test, again it showed that the composite having highest amount CNT (0.25%), was most thermally stable, as highest amount residue ( 27.28%) obtained after the decomposition temperature. The morphological studies were carried out by SEM. The prepared scaffolds have interconnected porous microstructures, which is convenient for bone growth in the scaffold. It was also observed that 0.1% MWCNT-composite showed least cytotoxicity and that of0.25. CNT showed highest toxic effects on cells. It is recommended that from the above results, (0.1%-0.15%) MWCNT- gelatin- chitosan-hydroxyapatite composite to use as artificial bone scaffold for enhanced physic-mechanical properties.
References
- KP Sanosh, Min-CheolChu, A Balakrishnan, TN Kim, Seong-JaiCho (2009) Utilization of biowaste eggshells to synthesize nanocrystalline hydroxyapatite powders. Materials Letters 63(24-25): 2100-2102.
- Eppley BL, Pietrzak WS, Blanton MW (2005) Allograft and alloplastic bone substitutes: a review of science and technology for the craniomaxillofacial surgeon. J Craniofac Surg 16(6): 981-989.
- Laurencin CT, Ambrosio AMA, Borden MD, Cooper Jr JA (1999) Tissue engineering: orthopedic applications. Annual review of biomedical engineering 1(1): 19-46.
- Jayachandran Venkatesan, BoMi Ryu, PN Sudha, Se-Kwon Kim (2012) Preparation and characterization of chitosan-carbon nanotube scaffolds for bone tissue engineering. International journal of biological macromolecules 50(2): 393-402.
- Bigham-Sadegh A, Oryan A (2015) Selection of animal models for pre-clinical strategies in evaluating the fracture healing, bone graft substitutes and bone tissue regeneration and engineering. Connect Tissue Res 56(3): 175-194.
- Lewallen EA, Riester SM, Bonin CA, Kremers HM, Dudakovic A, et al. (2014) Biological strategies for improved osseointegration and osteoinduction of porous metal orthopedic implants. Tissue Eng Part B Rev Part B: Reviews 21(2): 218-230.
- Gao C, Huo S, Li X, You X, Zhang Y, et al. (2007) Characteristics of calcium sulfate/gelatin composite biomaterials for bone repair. Journal of Biomaterials Science Polymer Edition 18(7): 799-824.
- Paulo Bartolo, Jean Pierre Kruth, Jorge Silva, Gideon Levy, Ajay Malshe, et al. (2012) Biomedical production of implants by additive electro-chemical and physical processes. CIRP Annals-Manufacturing Technology 61(2): 635-655.
- Garcia-Gareta E, Coathup MJ, Blunn GW (2015) Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 81: 112-121.
- Jayachandran Venkatesan, Zhong-Ji Qian, BoMi Ryu, Nanjundan Ashok Kumar, et al. Preparation and characterization of carbon nanotube- grafted-chitosan natural hydroxyapatite composite for bone tissue engineering. Carbohydrate Polymers 83(2): 569-577.
- Rashid TU, Rahman MM, Kabir S, Shamsuddin SM, Khan MA (2012) A new approach for the preparation of chitosan from Pa€irradiation of prawn shell: effects of radiation on the characteristics of chitosan. Polym Int 61(8): 1302-1308.
- Hsi-Kai Tsou, Hsueh-Chuan Hsu, Shih-Kuang Hsu, Shu-Ping Liou, Wen-Fu Ho (2013) A hydrothermal synthesis of eggshell and fruit waste extract to produce nanosized hydroxyapatite. Ceramics International 39(7): 8183-8188.
- KP Sanosh, Min-Cheol Chu, A Balakrishnan, TN Kim, Seong-JaiCho (2009) Utilization of biowaste eggshells to synthesize nanocrystalline hydroxyapatite powders. Materials Letters 63(24-25): 2100-2102.
- Zhongfan Liu, Ziyong Shen, Tao Zhu, Shifeng Hou, Lizhen Ying et al. Organizing single-walled carbon nanotubes on gold using a wet chemical self-assembling technique. Langmuir 16(8): 3569-3573.
- Nan X, Gu Z, Liu Z (2002) Immobilizing shortened single-walled carbon nanotubes (SWNTs) on gold using a surface condensation method. J Colloid Interface Sci 245(2): 311-318.
- Kai Yang, Mingyuan Gu, Yiping Guo, Xifeng Pan, Guohong Mu (2009) Effects of carbon nanotube functionalization on the mechanical and thermal properties of epoxy composites. Carbon 47(7): 1723-1737.
Editorial Manager:
Email:
biomedicalengineering@lupinepublishers.com

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...












