
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4579
Research Article(ISSN: 2637-4579) 
Performance Evaluation of Epoxy Coatings Containing Different Fillers in Natural and Simulated Environmental Conditions for Corrosion Resistance Volume 3 - Issue 4
Muhammad ANS1*, A Farooq2, MJK Lodhi2 and KM Deen2,3
- 1Ecole des Mines de Saint-Etienne, France
- 2Department of Metallurgy and Materials Engineering, University of the Punjab, Pakistan
- 3Department of Materials Engineering, University of British Columbia, Canada
Received: September 25, 2019; Published: October 10, 2019
*Corresponding author: Muhammad Ans, Department of materials science, Ecole des Mines de Saint-Etienne, France
DOI: 10.32474/OAJBEB.2019.03.000168
Abstract
Epoxy coatings are widely applied on steel structures due to their high adhesion strength and corrosion resistant properties. The transient environmental conditions and photo degradation could limit their long-term performance in many outdoor applications. The addition of different types of fillers could significantly change their barrier properties. In this study we investigated the corrosion performance of epoxy coatings on AISI 1040 steel after addition of different inorganic fillers i.e. TiO2, Fe2O3, ZnO and graphite according to ASTM G-50 and ASTM B-117 standards. The effect of various fillers on the adhesion and degradation behavior of epoxy coatings after exposure to atmosphere and saline solutions was estimated by visual inspection and Fourier transform infrared spectroscopy (FTIR). The significant loss in adhesion strength was observed after extended exposure to the ambient conditions. The notable changes in the intensity of IR signatures belonging to the primary amine, ethyl and origin of carbonyl group could be related with the degradation of coatings and were in agreement with the loss in adhesion strength. The relatively little change in the adhesion strength of ZnO containing epoxy coating compared to other fillers suggested its better performance.
Keywords: Epoxy coating; Degradable; Fillers; Salt spray analysis, Corrosion resistance
Introduction
Protective organic coatings are being widely used to protect steel structures and equipment form corrosion damages. Corrosion threat to commercial infrastructure is a monetary and engineering problem, while the expense of the coatings is a little part of the whole cost of the undertaking. The major victims of corrosion are chemical processing units, oil and gas industry, bridges, aircrafts, and marine industry which always invest their every possible resource to protect and to enhance the service life of their infrastructure [1]. The protective coating acts as a barrier between environment and substrate material, while it is an admitted fact that no ideal coating exist in reality. The performance of any coating in service mainly depends on the coating/substrate interface and its molecular structure which could limit the diffusion of electrolyte through it [2]. It is therefore to judge the quality of any coating, the adhesion strength with the substrate and its non-absorptive character towards electrolyte are the paramount [3]. The adhesive strength of the coatings with substrate is highly affected by the presence of contaminants including the dirt, chloride ions at the interface and/ or in the environment during service [4]. The presence of chloride, sulfate and nitrate at the surface of substrate could preferentially attract moisture at the interface by establishing continuous ions transport path through the coatings. This could lead to premature coating failure by blistering or complete delamination followed by sever corrosion of substrate [5,6]. Because of superior strength, chemical resistance and excellent adhesion strength to substrate, epoxy is termed as to be the most important class of anticorrosive paints with a service life of 50 to 60 years [7]. But at the same time epoxy is known for its susceptibility to degrade under exposure of sunlight and transient weather conditions. To reduce the vulnerable effects of these natural stimuli on the integrity of the epoxy coatings many different types of inorganic filler materials are added in them before application and curing. It is also well known that all polymeric films are permeable to oxygen and water, but in some cases the penetration of these species could exceed the tolerance limits during service and may initiate corrosion reactions at the coating/substrate interface [8]. Addition of inorganic fillers could also be beneficial in this respect to enhance their anti-corrosive properties [9]. Various type of inorganic filler materials has been used i.e. nano sized like ZrO2 [10], carbon black [11], Al2O3 particles [12] in the coatings to enhance their barrier properties to resist corrosion. The corrosion resistance can also be improved by coat the stainless steel with nano film of Al-Si [13]. In order to evaluate the performance of the coatings an analytical technique like FTIR is proved to be extremely helpful in the chemical characterization of the coatings [14]. This technique plays a vital role in evaluating the molecular change of the coatings during their exposure to the different environments. Adhesion is the key parameter that may determine the quality and durability of the coatings. Therefore, the focus of present work is to evaluate the effect of different fillers added to epoxy resin before application on the steel substrate. The influence of filler materials addition in the coating materials has been studied. The performance of these coatings under exposure to natural atmosphere and simulated conditions has been evaluated. The effect on the adhesion strength, photo stability and corrosion behavior have also been determined as a function of exposure time.
Experimental Work
The AISI-1040 steel panels were cut length=15cm, width=7.5cm from a sheet of 0.5mm thickness. The steel panels were grit blasted to remove mill scales and rusting followed by deburring before coating application. The substrate was placed at an angle of 45º with respect to the position of blasting gun in order to produce effective surface roughness. The surface tension of the prepared substrates was measured according to ISO-8296 standard by using DIN-53364 plasma ink, which turns out to be 38mN/m. A uniform layer of 60 μm zinc-sulphate primer was applied to achieve maximum adhesion strength of the coatings on the substrate surface. The coating material was prepared by mixing 78 (w/w) % epoxy resin, 17(w/w) % polyamide curing agent and 5(w/w) % of each TiO2, Fe2O3, ZnO and graphite as filler materials. The homogenous mixture was prepared by mechanical stirring at 600 rpm. To produce the desired consistency 1% acetone was also added in the mixture. To ensure uniform mixing of resin, curing agent, filler and other additives mixing was carried out for 15 min. These coatings were applied using roller brushes over pre-treated substrate panels and kept for 24 hours to dry and cure. These coated steel panels are designed as TiO2, Fe2O3, ZnO and graphite in the following discussion. The panels were exposed to natural atmosphere and to the saline environment in a salt spray chamber according to the ASTM G-50 [15] and ASTM B-117 standards [16] respectively. Three samples of each scheme were exposed to natural atmospheric condition; one sample from each scheme was taken after every 45 days for further testing and evaluation. The FTIR spectroscopy (IR Prestige-21, SHIMADZU Japan), adhesion tests ASTM D4541 (108 Hydraulic Adhesion Tester Elcometer) and visual examination according to ASTM G-33 standard were carried to estimate the integrity and corrosion behavior of coatings. The panels were placed in the salt pray chamber at 35 °C. The saline environment was created by maintaining, 5(w/v) % NaCl mist, at 1.3bars, under 100% humidity and pH 6.5 – 7.2 conditions. The panels were taken out after 500 and 1000 hours of exposure for further analysis according to the standard procedure.
Results & Discussion
Fourier Transforms Infrared Spectroscopy (FTIR)
FTIR Spectroscopy is a useful technique for molecular structural investigation of organic the coatings. The FTIR spectra present the interference fringes that may arise due to the constructive or destructive interference from the refracted functional groups, giving characteristics peaks to evaluate structural information. Figure 1 shows the FTIR spectra of coated steel samples after different exposure to natural atmosphere according to ASTM G-50 containing different fillers i.e. TiO2, Fe2O3, ZnO and graphite, respectively. It was observed that each coated sample presented vibrational bands at (2800-2990cm-1) corresponding to –CH3 functional group. The stretching vibrations related with the OH (3100- 3600cm-1) was also observed. The peaks at 1580-1650cm- 1, 1230cm-1, 1000-1100cm-1and at 830cm-1could be associated with the primary amine (NH), ETHER, C-N group and epoxy ring structure, respectively [17-19]. After exposure, the peak at wave number (1720cm-1) was originated which could be attributed to the stretching of carbonyl functional group. This could be due to the reaction of atmospheric oxygen with the free radicals at the surface of coating [20]. The epoxide group in the resin interacts with the amines functional groups of polyamides curing agent during curing process as presented in reaction scheme Equation I-IV [21].
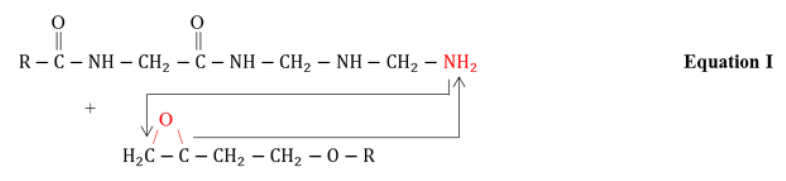
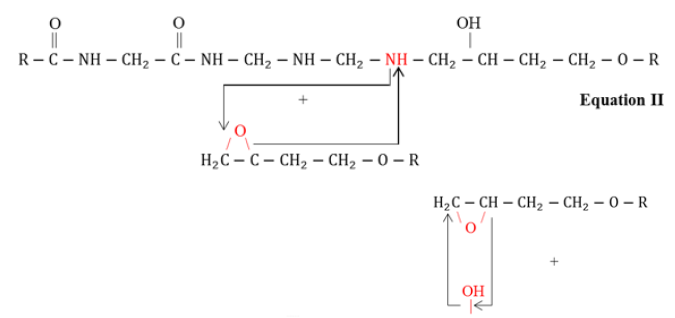
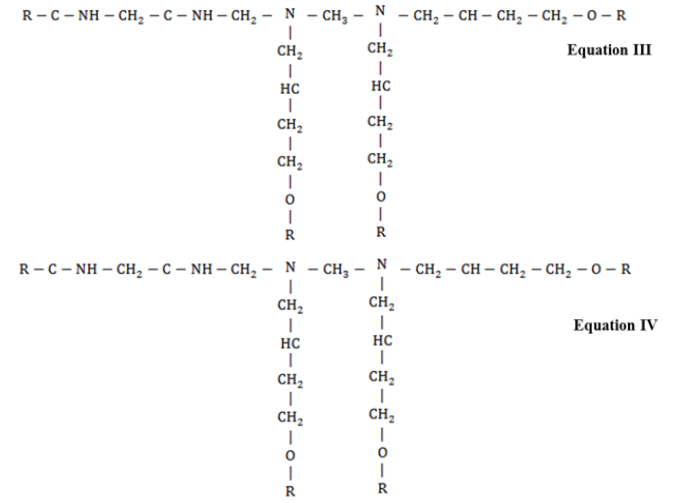
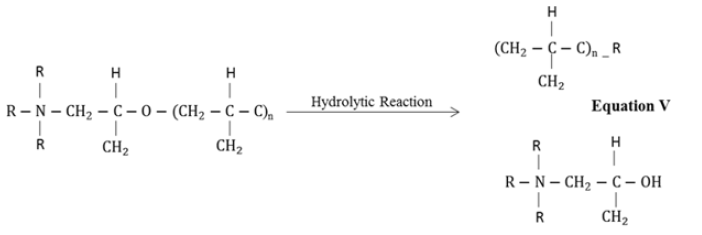
Briefly, in the first step, the amine (–NH2) group present at the end of polyamide curing agent chain opens up the epoxy ring followed by chain extension and branching in the three dimensional network structure. In addition, formation of hydroxyl groups (–OH) during curing process and decrease in concentration of epoxy rings represent the effective cross linking and complete curing of resin. Also, the decrease in the concentration of primary amines (present on the polyamide chains) could also be associated with the curing of coatings. In service the moisture and other aggressive ions in the environment could interact with the hydrophilic functional groups i.e. –OH, =C=O and –NH– groups and may promote the degradation of coatings. The absorption of water in the coating would also be a function of exposure time but the nature of filler compounds in the coating could significantly alter its performance characteristics. It was evaluated that upon extended exposure, the decrease in the intensity of =C–O– functional group (1230cm-1) and increase in the band intensity associated with vibration of –OH functional groups was observed. The increase in the intensity of hydroxyl groups could be associated with the absorption of water or hydrolytic degradation of coating molecular structure. Compared to other fillers, the ZnO based coating presented negligible decrease in the etheric group and small change in the broad band (≈3300cm-1) associated with the –OH groups. This behavior could be attributed to the beneficial effects of ZnO in the epoxy coating. In other filler containing coatings the increase in the intensity of –OH functional group was observed. The decrease in the etheric band and increase in the OH band intensity could be associated with the hydrolytic degradation of the epoxy coating as shown in Equation V [22].
Figure 1: Transmission spectra of epoxy-based coatings containing different filler material (a) TiO2 (b) Fe2O3 (c) ZnO (d) graphite.
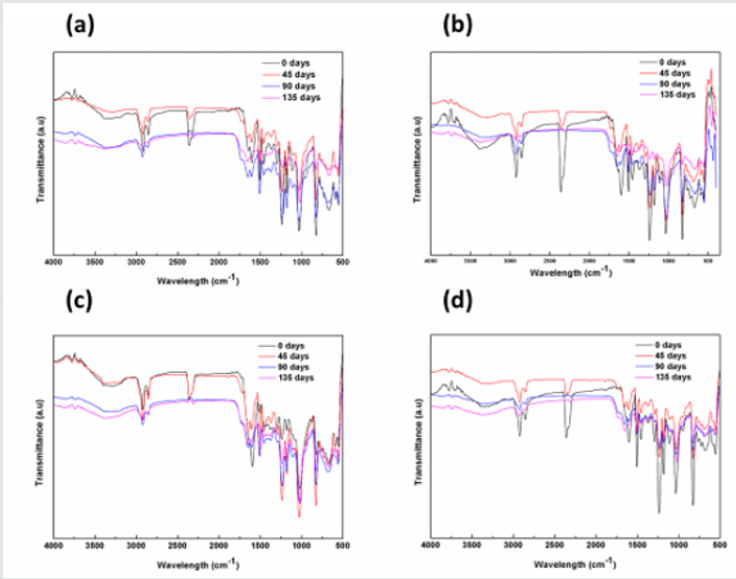
In hydrolytic degradation, the ether bond in the epoxy polymeric chain could break along with the formation of hydroxyl group [23]. Based on the limited increase in the –OH intensity and small change in the intensity of etheric group could be associated with the limited hydrolytic degradation of ZnO containing coating. In case of graphite and Fe2O3 filler containing coatings, the considerable loss in the intensity of primary amine could be observed after extended exposure in the natural atmospheric conditions. This could possibly be related with the adverse atmospheric conditions in synergism with photo-degradation of polymeric chains. The TiO2 containing coating started to show loss in intensity after 90 days incubation period in the natural environment. This loss in the intensity associated with the primary amine groups which could also be related with the opening up of the epoxy rings. In initial 45 days of exposure, the graphite and Fe2O3 containing coatings presented decrease in the intensity of primary amine peak and etheric group compared to TiO2 coating which may inhibit deterioration during this time. The peak at 2800cm-1which corresponded to the methyl functional group, the gradual decrease in the intensity was observed in case of graphite, TiO2 and Fe2O3 coatings compared to ZnO containing coating in the initial 45 days exposure.
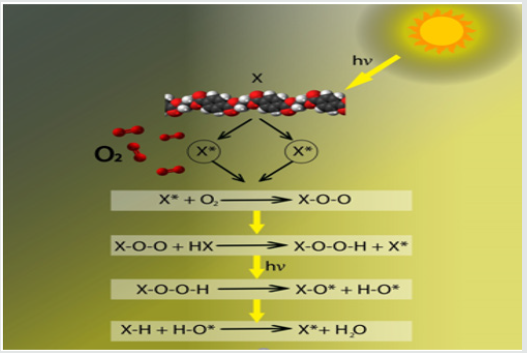
Under ambient conditions, the ultraviolent radiations could also degrade the primary bonds of the polymeric structures by generating free radicals and the process called photolysis is initiated The free radicals produced by photolysis could react with atmospheric oxygen to form per-oxy radicals followed by dissociation into free radicals and water as shown schematically in Figure 2. These reactions lead to the phenomenon of chain scission, removal of small polymeric materials and de-polymerization etc. [24]. In epoxy-based coatings methyl group of the main chain are more vulnerable to degradation under ultraviolent radiations and could be converted into carbonyl [25]. The FTIR results validated the decrease in the intensity of methyl functional group with the generation of carbonyl peak at 1720cm-1after initial 45 days exposure in natural environment. This behavior could be attributed to the degradation of polymeric chains into free radicals after exposure to natural sunlight and atmospheric oxygen [17,20]. The carbonyl group belongs to chromo-phoric group, which may absorb energy from sunlight and could excite the electrons leading to reaction with the oxygen. All coating systems except ZnO containing coating system represented the formation of carbonyl group and the increase in the intensity could be used as indicator to coating degradation under extended exposure to natural environment. The TiO2, graphite and Fe2O3 containing coating systems represents prominent signatures of carbonyl functional group at 1720cm- 1wave number which appear in the initial 45 days of exposure, but the origin of this peak delayed for 90 days in case of ZnO containing coating system. These behaviors could be elucidated as the limited photolysis of the coating system containing ZnO as filler. This beneficial effect of ZnO as filler could be related with its relatively wider band gap (3.37eV) and therefore could absorb large amount of UV radiation energy under atmospheric conditions [26]. However, the graphite and Fe2O3 based coatings provided least resistance to photo-degradation under same conditions.
Figure 2: Where X= group of polymer before irradiation, X* = polymer alkyl radical, X–O* = polymer oxy radical, X–O–O = polymer per-oxy radical, X–O–O–H = polymer hydro peroxide, H–O* = hydroxyl radical.
Adhesion test
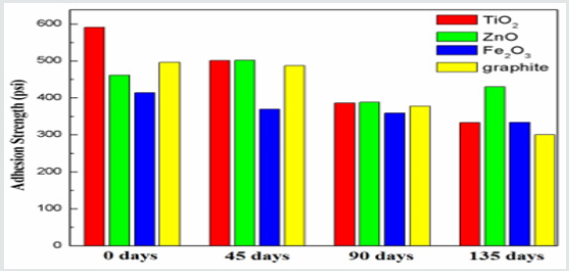
The mechanical integrity of the coating system is a function of coating/substrate bonding strength. It is therefore, the adhesion strength of theses coating systems after seasoned in the natural environment was measured after different exposure time periods as shown in Figure 3. Visually, the coatings did not represent any signs of delimitation from the substrate even in the presence of moisture, sunlight and humidity at variable ambient conditions of outdoor atmosphere [27]. The adhesion strength of un-exposed coated samples (as prepared) was also measured for comparison as given in Figure 3. Although the graphite-based coatings presented high UV stabilization but significant decrease in the adhesion strength after exposure corresponded to the agglomeration of the filler particles into clusters possibly by the weak Van Der Walls attraction between the particles [28]. After 90 days of exposure, the decrease in the adhesion strength was observed for all the coating schemes and this trend continue for even 135 days of exposure except the ZnO containing coating. It was evaluated that the decrease in the adhesion strength also depends on the nature of the filler materials used in the coatings. In case of ZnO containing coatings, the relatively minor change in the peak intensity associated with methyl group without any evidence of carbonyl functional group demonstrated the better stability even after extended exposure (135 days). The ZnO is known for its effective ultraviolent light absorption capability. This could absorb even shorter wavelengths UV radiation <360nm and therefore provided better protection against ever changing environmental conditions. However, TiO2 could be reduced at smaller wavelength (<405nm) by producing nascent oxygen and could oxidize the epoxy by forming water soluble breakdown products [27]. ZnO is recognized for its better UV radiation absorption competency, which could possibly decrease the degradation of epoxy polymeric chains under sever atmospheric conditions in our case.
Figure 3: Comparison of adhesion strength for different fillers-based coatings for different exposure time.
Salt spray
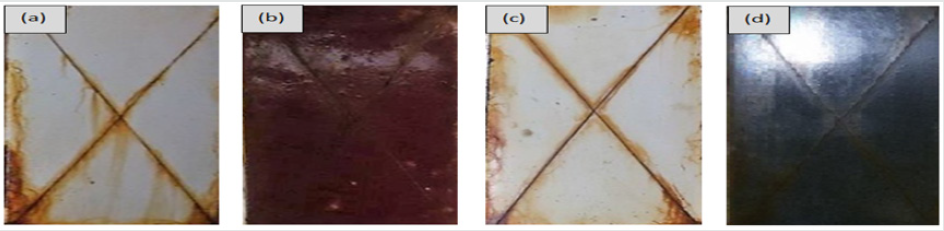
The corrosion behavior of the coated samples was investigated by exposing them in the salt spray chamber, for 1000 hours. According to ASTM-B-117, the bath was kept at 35 °C and 5% NaCl containing mist was sprayed at 1.3 bars pressure. The environment in the chamber was maintained as 100% relative humidity and pH 6.5 – 7.2 was pre-adjusted. After 500hrs. of exposure in the salt spray chamber the samples were taken out to measure the adhesion strength and for visual examination. The tape method was used to measure the adhesion strength on the coatings (ASTM D-3359). After 500 hrs. exposure, the coated samples showed adequate adhesion without delaminating from the metallic substrate. No pitting or local damage was observed, and the coating gloss was negligibly affected. The adhesion tests according to ASTM D-3359 standard were performed after 1000 hours exposure in the salt spray chamber. No signs of blistering or delamination were observed even after 1000 hours of exposure in the aggressive environment and the edges of the hatch were observed smooth as shown in Figure 4. The slight discoloration in the graphite containing coatings was observed without any blistering however, the hatches made on the coated panels were rust filled and stained as brown product due to localized corrosion and flow of aqueous saline solution over the panels after condensation [28-31]. No lifting or de-lamination was observed adjacent to the scribe line which also indicated the good adhesive characteristics of the epoxy coating with the steel.
Figure 4: Samples after 1000 hours of exposure in salt spray chamber (a) ZnO (b) Fe2O3 (c) TiO2 (d) graphite.
Visual inspection of the samples exposed to the atmosphere
Initially, after 45 days of exposure to the natural atmospheric conditions the samples were taken for visual inspection. The exposed samples did not give any obvious sings of degradation or any loss in gloss for ZnO and TiO2 containing coated samples whereas, the Fe2O3 and graphite coated samples exhibited a slight varying degree of discoloration. The epoxy coating is considered good for outdoor application due to its better discoloration and chalking properties. The ZnO and TiO2 containing coatings presented limited discoloration compared to graphite and Fe2O3 coatings. No blisters, surface defects or localized corrosion spots were evident in these coated panels even after 90 days of exposure under transient outdoor environmental conditions. Slight loss in gloss was observed for TiO2 containing coating after 135 days exposure without any delamination or local damage.
Conclusion
The degradation of epoxy coatings containing various filler materials were investigated under natural and accelerated environmental conditions. Based on the experimental results and visual observations the following conclusions were drawn.
a) The FTIR analysis depicted that the methyl group to be
known as the photo-initiating group which could exhibit change
in the vibrational intensity based on the environmental effects.
The relatively large decrease in the peak intensity associated
with the methyl group indicated greater degree of degradation
in the coatings. ZnO containing epoxy coatings showed more
pronounced UV stabilization characteristics which could
extend the service-life of the epoxy coating compared to other
filler materials exposed to same environmental conditions.
b) The relatively minor decrease in the adhesion strength
by ZnO containing coatings indicated its better mechanical
integrity and UV resistant characteristics compared to coatings
containing TiO2, Fe2O3 and graphite filler materials.
c) The addition of antioxidants and photo-stabilizers in the
epoxy coatings showed excellent resistance during aggressive
salt spray test with negligibly minor loss in the adhesion
strength without any blistering formation.
Acknowledgement
The authors wish to express their thanks for the financial support of grants to University of the Punjab, Lahore and Ecole des Mines, France.
Conflict of Interest
The authors declare no conflict of interest.
References
- Peabody AW (2001) Peabody's control of pipeline corrosion. NACE (2nd edn).
- Hare CH (1986) Adhesive and Cohesive failure in applied coating system composites. JPLC p. 38-48.
- Bellucci F, Nicodemo L (1993) water transport in organic coatings. Corrosion 49(3): 235.
- Mayne JEO (1959) The problem of painting over rusty steel. J Appl Chem 9(12): 673-680.
- Dela Fuente D, chico B, Morcillo M (2006) The effect of soluble salts at the metal/paint interface: Advances in knowledge. Portugaliae Electrochemia Acta 24: 191- 206.
- MCHMMMD de la Fuente (2007) The settling of critical levels of soluble salts for painting. Prog Org Coat 23-32.
- KICCLTJR (2000) Given Fusion Bonded Epoxy-Failure Modes and Effects Analysis- Prelimary Survey.
- Mayne J (1973) The mechanism of the protection of iron and steel by paint, Anti-corros. Methods Mater 20: 3-8.
- Wicks FSPZW (1992) Organic coatings in Science and Technology Wiley. New York, USA.
- Behzadnasab SMMEM (2013) Corrosion Protection of steel by epoxy nanocomposite coatings containing various combinations of clay and nano particulate Zirconia Corrosion Science 75: 134-141.
- Ahmad Ghasemi Kahrizsangi HSJNEA (2015) Degradation of modefied carbon black/epoxy nanocomposite coatings ultraviolet exposure. Applied Surface Science 353: 530-539.
- PPSHRN Jagtap (2008) Prog Org Coat 63: 389-394.
- Muhammad ANS (2019) Nano-mechanical properties of novel intermetallic coatings on 316L bioimplant material Biomaterials. Tissue Engineering Bulletin pp. 119-127.
- Maria Gonzalez Material science Engineering and Technology Spain pp. 261-286.
- (2015) standard practice for conducting Atmospheric Corrosion Test on Metal, ASTM international.
- (2019) Standard practice for operating salt spray (fog) Apparatus. ASTM international.
- CCLRD Rosu (2006) Effect of UV radiation on photolysis of epoxy maleate of bisphenol," AJ Photochem. photobiol. A Chem pp. 218-224.
- GMM Avram (1972) Infrared Spectroscopy Applications in Organic Chemistry. J Wiley & Sons, New York, USA.
- SMCCL ZRG Nikolic (2010) Fast Fourier transform IR Chracterization of epoxy GY system crosslinked with aliphatic and cycloaliphatic EH polyamine adducts, sensor 10: 648-696.
- LZSSJ Simitzis (2002) Effect of composition and polyesterification catalysts on the optical properties of cutred poyesters. Polym Int p.318.
- Brand Jd (2004) On the adhesion between aluminium nand polymers.
- Ramezanzadeh B, Khazaei M, Rajabi A, Heidari G (2013) Corrosion Resistance and Cathodic Delamination of an Epoxy/Polyamide Coating on Milled Steel. Corrosion Science 70(1): 56-65.
- SSHSF (1989) Mansfeld Corrosion 45: 615-630.
- Hare CH (1992) The Degradation of Coatings by Ultraviolet Light and electrochemical radiation. joural for protective coating and lining.
- Asmatulu GMCHHMR (2011) Effect of UV degradation on surface hydrophobic, crack and thickness of MWCNT-based nanocomposite coatings. Prog Org Coat 72(3): 553-561.
- Sobana MN (2007) The Effect of Operational Parameters on the Photocatalytic degradation of acid red 18 by ZnO. Separation Purification Technology 56(1): 101-107.
- Liu HM (2002) Effect of Carbon Black on UV stability of LLDPE films under artificial weathering conditions. Polym Degrad Stabil 75(3): 485-499.
- Norman D (2004) Excellent pipeline coatings require excellent pipeline substrate. NACE Corrosion.
- Popovic BGVMSSMM (2005) Corrosion studies on electrochemically deposited OANI and PANI/epoxy coatings on mild steel in acid sulphate solution prog Org Coat 52: 359-365.
- Simitzis LSSJ (2002) Effect of composition and polyesterification catalysts on the optical properties of cured polyesters. Polym Int pp. 308-318.
- Kumar RSiTNBG (2002) Degradation of carbon fiber-reinforced epoxy composites by ultraviolet radiation and condensation. J Compos Mater pp. 2713-2721.
Editorial Manager:
Email:
biomedicalengineering@lupinepublishers.com

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




