Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4579
Research Article(ISSN: 2637-4579) 
Elemental Analysis of Vitexaltissimalinn. leaves by X-ray Fluorescence and its Biological Implications and Studies Volume 4 - Issue 1
Sunitha S1, Daisy Joseph2*, Lekshmi V Kumar1 and RathikaNath G1
- 1Department of Chemistry and Polymer Chemistry, K.S.M.D.B College, Sasthamcotta (Research Centre, University of Kerala) India
- 2Nuclear Physics Division, Bhabha Atomic Research Centre, Trombay, Mumbai, India
Received:March 09, 2020; Published: July 16, 2020
*Corresponding author: Daisy Joseph, Nuclear Physics Division, Bhabha Atomic Research Centre, Trombay, Mumbai, India
DOI: 10.32474/OAJBEB.2020.04.000180
Abstract
Elemental analysis of V.altissima (L) leaf was carried out by using X-ray fluorescence spectrometer and SEM-EDX. Fifteen of the detected elements are essential, which includes some heavy elements (Al, Si, P, K, Ca, Mn, Fe, Cu, Zn, Sr, Nb, Mo, Cd, Th and U) while 20 others (Mg, S, Ti, V, Cr, Co, Ni, As, Se, Rb, Y, Zr, Ag, Sn, Sb, Ba, W, Hg, Pb and Bi) were found to be below detection limit in Hand held X-ray Fluorescence (HHXRF) analysis. From SEM-EDX results, non-transition elements such as C, O, Si, S, K, Ca and Br were found to be present in the dried leaf powder of V.altissima. The distribution of elements was found to be in the order: crude leaf powder> Hexane> Chloroform> Methanol. From the results it is clear that V. altissima contain many essential elements which are all required minerals for human Biological studies such as DPPH scavenging assay and reducing power was carried out on these plant extracts to evaluate their anti-oxidant capacity. It was seen that these plant extracts do have a good anti-oxidant levels.
Introduction
Medicinal plants are rich sources of bioactive components which play important role in the prevention of variety of diseases. Vitexaltissima belonging to the family Verbenaceae, is a moderate to large sized tree [1]. Leaves of the plants are reported to use for the treatment of rheumatism [2]. Antioxidant and anti-inflammatory activities of the plant leaves are also reported [3]. Phytochemical screening of secondary metabolites present in dry leaves and their percentage of yield was also calculated. Phytochemical analysis revealed the presence of flavonoids, terpenoids, and spooning in dry leaf extracts. Isolation is the main step in Photochemistry, where the biologically active compounds are separated in its purest single form. This process was carried out using repeated column chromatography, whereby a single biologically active compound was separated. This isolated compound was identified with the help of its spectral data, such as IR, 13CNMR, 1H NMR and mass spectra.
Photochemical analysis of V.altissima leaf extracts reveals the presence of triterpenes, phenol acids, steroids, coumarone, flavonoids, fatty acids etc. These secondary metabolites were reported to play a major role in various biological activities, which explains its use as a traditional medicine. An increased scientific interest is noticed presently on modern medicine and herbal products. Medicinal plants have a prominent role in the pharmaceutical industry of 21st century [4, 5, 6].
Various essential and non-essential metals are also present in plants in addition to the secondary metabolites. The higher and lower concentrations of these trace metals may cause metabolic disturbances [7] These metals may include both essential micronutrients and toxic metals, the deficiency and excess of which may cause serious effects on human health [8, 9]. Hence, the safety of the herbal products and its use has recently been questioned due to the reports of illness and causalities [10]. The intake of heavy metal by human may cause disturbances of normal functions of brain, kidney, lungs, heart, liver etc. and leads to ulcers and different types of cancers [11].
Experimental
Collection of plant material and its Extraction
Vitexaltissima leaves were collected from Jawaharlal Nehru Tropical Botanic Garden and Research Institute (JNTBGRI), Palode, Thiruvananthapuram, Kerala state, India. V. altissima leaves were cut into small pieces, and shade dried and powdered using a cross beater mill. V. altissima leaf powder (520 g) was subjected to sequential extraction on Sox let apparatus with hexane, chloroform and methanol (2.5 L each) successively (24 hr, each). The extracts were filtered and concentrated under reduced pressure using a rotary evaporator. Percent yields of each extracts were noted
The methanol extract, obtained after sox let extraction is then subjected to liquid-liquid partition and again into two on the basis of the polarity of the solvents. The methanol extract was dissolved in minimum quantity of water, which was then made up to 200ml with rest of water. Equal amount of ethyl acetate was added to it and mixed in a separating funnel, allowing to separate into aqueous and organic layers. Water containing aqueous layer is the lower part of the separating funnel and the upper layer is ethyl acetate containing organic part. The upper organic part was collected and concentrated. The procedure was repeated several times each time with 200ml of ethyl acetate, until the organic layer has no color. After washing using ethyl acetate, same procedure as repeated with butanol. The collected ethyl acetate, butanol and aqueous water fraction were concentrated and dried with rotary evaporator for further studies.
Results and Discussion
altissima leaf powder (520 g) was extracted with three solvents of increasing polarity namely hexane, chloroform and methanol. The extracts were concentrated under reduced pressure using a rotary evaporator. The percentage of yield for each extract is that 2.82, 6.01 and 11.5 (weight of each extracts are 14.72, 31.333 and 60.05). The yield of methanol extract was found to be high compared to hexane and chloroform extracts.
Phytochemical screening of extracts
The extracts (hexane, chloroform and methanol) obtained after sox let extraction were subjected to photochemical screening using standard procedure. Results show that hexane extract is enriched with steroids while chloroform is carbohydrate rich extract. Because of higher polarity of flavonoids and spooning, methanol extract produces positive result for both of them.
Elemental studies of various extracts of V.altissima leaves
All these extracts were analyzed for trace elements using HHXRF
and SEM-EDX using the crude samples. Hand held XRF (HHXRF) is
the need of the hour to analyze metals, powders and alloys, as other
conventional XRF techniques were found to be cumbersome and
difficult to handle.
The HHXRF was directed at the samples and irradiated
using X-ray tube. Rhodium tube the spectrum was obtained in 60
seconds. A Typical spectrum of leaf extract is shown in (Figure1).
The prominent peaks of K (3.3 keV) and Ca (3.6KeV) are seen. The
beam lines were from 12 to 36 keV (Beam 1) and from 0 to 12 KeV
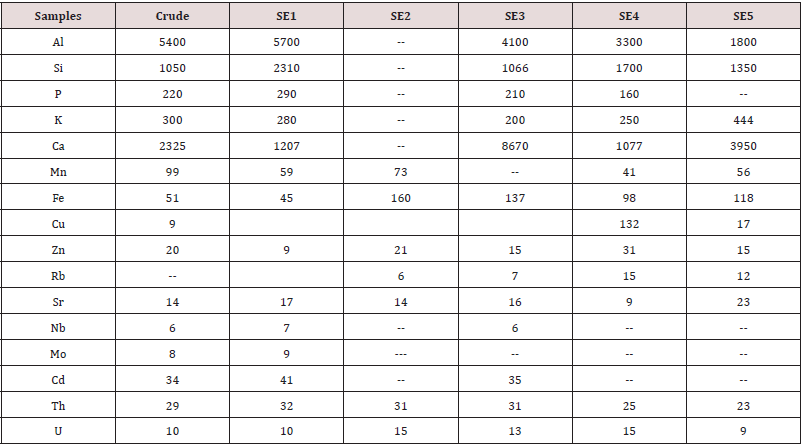
(Beam 2) (Figure 2, Table 1).
*crude- dried leaf powder; SE1-Hexane extract; SE2- Chloroform extract; SE3- Ethyl acetate extract; SE4-Butanol extract; SE5- Water extract. (**SE- Solvent extract) Al, Si, P, K, and Ca are seen to be present significantly which are useful elements. Al which cannot be detected by many conventional instruments in XRF due to low energy X-ray absorption of detector window could be detected by HHXRF. HHXRF has SDD (Silicon drift detector) which has a grapheme window and the Al X-rays do not get absorbed unlike in the Si (Li) (Lithium drifted). Silicon X-ray detector which has Be window, absorbs low energy X-rays below Z<19. Thus, HHXRF can be a useful tool for detecting Al in samples. There are no toxic elements in the sample except Cd, which is very below MPL. The role of each of these elements needs to be investigated and the study is underway for further conclusions.
DPPH Radical Scavenging Assay
The radical scavenging activity of different extracts was determined by using DPPH assay according to Chang et al. [12]. The decrease in the absorption of the DPPH solution after the addition of an antioxidant was measured at 517 nm.
Principle
1, 1-diphenyl-2-picryl hydroxyl is a stable free radical with
pink color which turns yellow when scavenged. The DPPH assay
uses this character to show free radical scavenging activity. The
scavenging reaction between (DPPH) and an antioxidant (H-A) can
be written as,
DPPH + [H-A] →DPPH-H + (A)
Antioxidants react with DPPH and reduce it to DPPH-H and as consequence the absorbance decreases. The degree of discoloration indicates the scavenging potential of the antioxidant compounds or extracts in terms of hydrogen donating ability
Reagent Preparation
0.1mM DPPH solution was prepared by dissolving 4mg of DPPH in 100ml of methanol.
Procedure
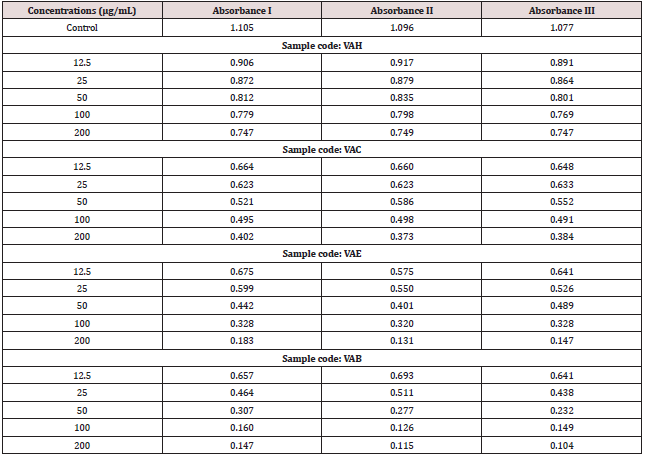
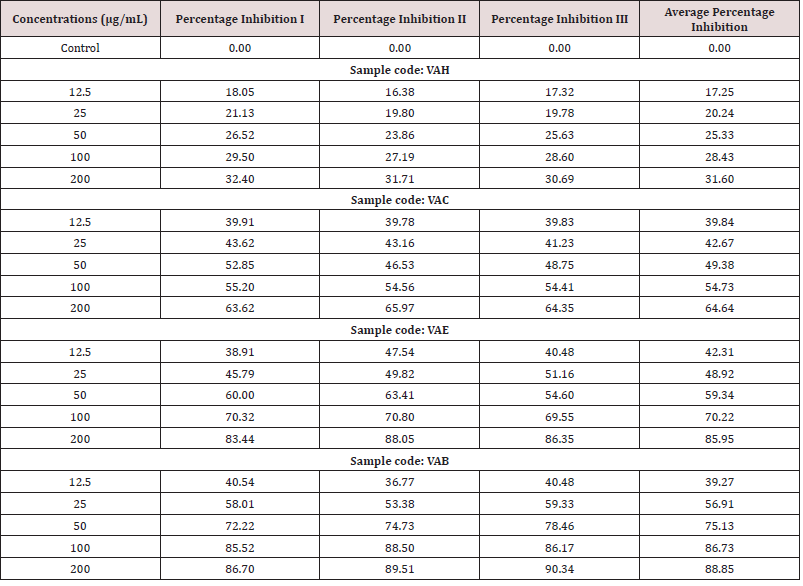
Different concentrations of sample such as 12.5μg/mL- 200μg/ mL from stock solution were made up to a final volume of 20μl with DMSO and 1.48ml DPPH (0.1mM) solution was added. The reaction mixture incubated in dark condition at room temperature for 20 minutes. After 20 minutes, the absorbance of the mixture was read at 517nm. 3ml of DPPH was taken as control.
Calculation
 (2)
(2)
Results
(Tables 2,3)
Table 3: IC50 Value- VAH-440.173μg/mL (Calculated using ED 50 PLUS V1.0 Software). VAC-75.522μg/mL; VAE- 28.7433μg/mL; VAB-17.8732μg/mL.
Reducing Power Activity
The reducing power of extract was determined by the method of YEN and DUH (1993).
Procedure
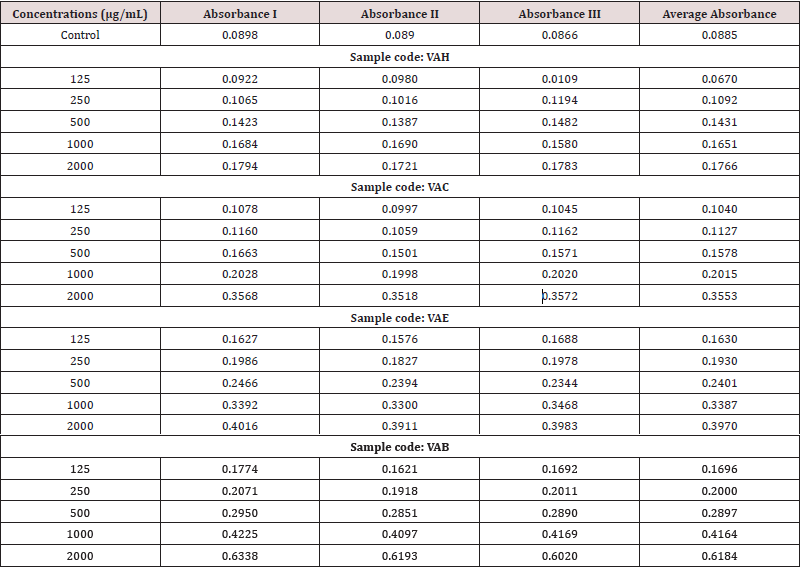
Different concentrations of sample such as 125μg/mL-2000μg/ mL from a stock concentration of 10mg/mL were mixed with 2.5ml of phosphate buffer (200mM) (pH 6.6) and 2.5ml of 1% potassium ferric cyanide was added and boiled for 20 minutes at 50°C. A control without the test compound, but an equivalent amount of distilled water was taken. After incubation, 2.5 ml of 10% TCA were added to the mixtures followed by centrifugation at 650g it for 10 minutes. The upper layer (5ml) was mixed with 5ml of distilled water and 1ml of 0.1% ferric chloride was added and absorbance was read at 700nm.
Results
(Tables 4)
Conclusions
A vast amount of research is on way to explore various herbal products. In addition to various primary and secondary metabolites, photochemical include a variety of essential and non-essential elements. Thus, the safety use of herbal products lies on its content of trace elements also. Photochemical analysis of V.altissima leaf extracts reveals the presence of triterpenes, phenol acids, steroids, coumarone, flavonoids, fatty acids etc. In the present study, the leaf extract was estimated to contain several essential elements. This knowledge about the elemental composition will be useful while formulating drugs for specific purposes.
Acknowledgements
We wish to acknowledge Dr. Celin Acharya, PallaviJoshi, MBD, BARC for their help in the SEM_EDX analysis. We are also thankful to the Director and HOD of Jawaharlal Nehru Tropical Botanic Garden and Research Institute (JNTBGRI), Palode, Thiruvananthapuram for providing all facilities for the extraction procedure.
References
- Pullaiah T, Sandhyarani S (1999) Trees of Andhra Predesh India. Regency pp. 375-376.
- NarayanaRao K, Thammanna T (1990) Medicinal Plants of Thirumala. TTD, Tirupati, India p. 57.
- Sridhar C, Subbaraju GV, Venkateswarlu Y, Raju TV (2004) New acylatediridoidglucosides from Vitexaltissima. J Nat Prod 67.
- Khan MSY, Bano S, Javed K, Mueed MA (2006) A comprehensive review onthe chemistry and pharmacology of Corchorusspecies: A source of cardiacglycosides, triterpenoids, ionones, flavonoids, coumarins, steroids and someother compounds. Journal of Scientificand Industrial Research 65: 283-298.
- Delang CO (2007) The role of medicinal plantsin the provision of healthcare in Lao PDR. Journal of Medicinal Plants Research 1(3): 50-59.
- Hosseinzadeh S, Jafarikukhdan A, Hosseini A, Armand R (2015) The Application of Medicinal Plants in traditional and Modern Medicine: A review of Thymusvulgaris. International Journal of ClinicalMedicine 6: 635-642.
- Chopra RN, Chopra IC, Honda KL, Kapoor CD (1958) Indigenous drugs of India (UN Dhar and Sons, Kolkatta). Pp. 501– 502.
- Satyavati GV, Raina MK, Sharma M (1976) Medicinal plants of India ICMR. New Delhi 1: 278-281.
- Wahid A, Siddiqui HH (1961) A survey of Drugs (2nd). Published by IHMMR, Delhi 151: 4a.
- Gupta LM, Raina R (1998) Side effects of some medicinal plants. Current Science 75(9): 897-900.
- Jaishankar M, Tsten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effect of some heavy metals. Interdisplinary Toxicology 7(2): 60-72.
- Chang ST, Wu JH, Wang SY, Kang PL, Yang NS, eta l. (2001) Antioxidant activity of extracts from Acasia confuse bark and heartwood. J Agric Food Chem 49: 3420-3424.
Editorial Manager:
Email:
biomedicalengineering@lupinepublishers.com

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...