Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2690-5752
Review Article(ISSN: 2690-5752) 
Reconstruction of African Bipedal Primates’ Phylogeny Based on the Adaptive Species Axiom Volume 5 - Issue 2
Sergey V Vyrskiy*
- Russia
Received:August 27, 2021 Published: September 13, 2021
Corresponding author: Sergey V Vyrskiy, E-mail: sergey.vyrskiy@gmail.com, ph.No: +79173224329
DOI: 10.32474/JAAS.2021.05.000207
Abstract
Paleoanthropology is yet to come up with a generally accepted definition of a species as a basic unit for classification of evolving groups of individuals. It causes an excessive number of assigned species and unjustified splitting of hierarchical levels of classification that impedes adequate reconstruction of their phylogeny. This research studies the species characteristics posited by C. Linnaeus and supplemented by Ch.R. Darwin’s theory of descent with modification to suggest an adaptive species axiom, as well as instrumental methods of its diagnostics and differentiation. The application of the proposed methods has demonstrated only two adaptive species of bipedal primates existing 6-1 mya. Presumably, they were formed 9-8 mya as a result of branching of a maternal species that already possessed bipedalism. Both of these species became extinct, but the population of one of them formed a new species 2.6 mya with its industrial activity and natural resources resembling those of homo sapiens.
Keywords: Phylogeny; African Bipedal Primates; Adaptive Species; homo sapiens; Paleoanthropology
Introduction
The paleoanthropology of the African continent started in 1913 with the research of Olduvai Gorge, Tanzania, where the team of Professor Hans Gottfried Reck from Humboldt University of Berlin discovered a partial skeleton of a bipedal primate from deposits aged 1.5-0.4 mya (Reck,1914). Unfortunately, this skeleton was not studied sufficiently and was later lost, and thus the unique discovery went to oblivion.
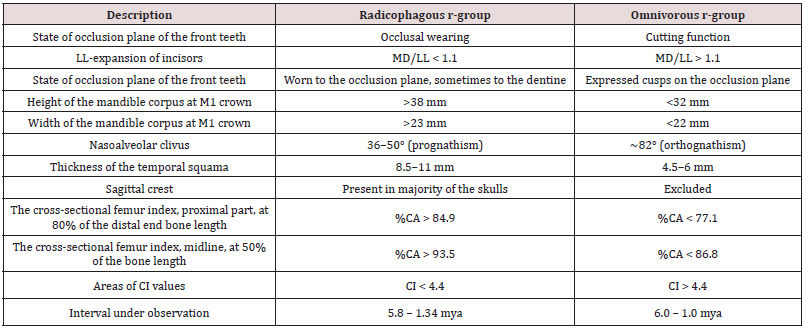
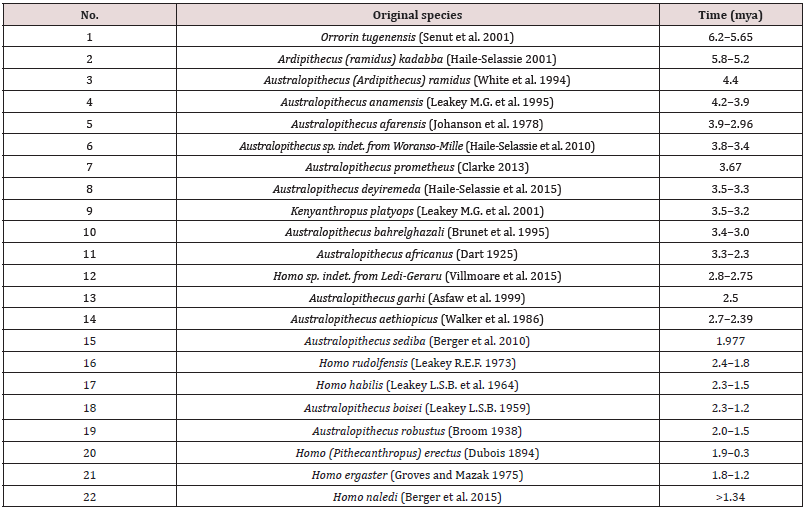
Nowadays, there are 16 described species of bipedal primates in five genera with a total of six species of the homo genus in African deposits dated 6.0-1.0 mya (Table 1).
Table 1: The list of original bipedal primate species and early homo from the African deposits 6.0-1.0 mya.
Meanwhile, there are 6 more intermediate hierarchical categories within the classification between Order Primates (Linnaeus 1758) and Genus homo (Linnaeus 1758):
Order Primates Linnaeus, 1758
Suborder Anthropoidea Mivart, 1864
Superfamily Hominoidea Simpson 1931 (Superfamily Hominoidea Gray, 1825)
Family Hominidae Le Gros Clark 1955 (Family Hominidae Gray, 1825)
Subfamily Homininae™ Delson & P. Andrews, in Luckett & Szalay, eds., 1975
Tribe Hominini Gray 1825 (Gray, 1825)
Subtribe Hominina™ Delson & P. Andrews, in Luckett & Szalay, eds., 1975 (Gray, 1825)
Genus homo Linnaeus, 1758
However, this classification is constantly amended with some categories either reassigned or excluded from the nomenclature, or the hierarchical levels changed altogether. There were originally 11 genera of bipedal primates but 6 of them, namely Paranthropus, Plesianthropus, Zinjanthropus, Praeanthropus, Paraustralopithecus, and Meganthropus were later recognized as unavailable and changed. However, [Richmond B.G. at al., 2020] assigned upper limb skeleton KNM-ER 47000 to Paranthropus boisei, while [Martin J.M. at al., 2021] referred cranium DNH 155 to Paranthropus robustus, thus returning the previously rejected genus Paranthropus to scientific practice.
[E. Mayr 1969] mentioned different proposals to include as much as 30 generic names into the Family Hominidae.
For example, genus Australopithecus was first included into the Family homo-simiadae [Dart 1925], then into the Family Hominidae [Johanson et al. 1978], then into the Superfamily Hominoidea [White et al. 1994], and then into the suborder Anthropoidea [Asfaw et al. 1999] until [Berger et al. 2010] returned it into Hominidae, and [Haile-Selassie et al. 2015] assigned it to the Superfamily Hominoidea.
The bipedal primates from the South African sites of Taung, Sterkfontein and Makapansgat originally got three different species names of А. africanus, A. transvaalensis, and A. prometheus, and a common phylogenetic name of an ape-man. Subsequently, all species were united under the name of A. africanus, though [R.J. Clarke 2013] returned the name A. prometheus to partial skeleton StW 573 (Little foot) found in Sterkfontein.
From the phylogenetic point of view, it might be concluded that approximately 6.0 mya the continent was bound to witness an evolutionary “burst”-either a transition of some arboreal primates to bipedal walking, or numerous branching of one bipedal species. However, the absence of transitional (intermediate) forms of individuals does not support this assumption.
Excessive hierarchical splitting and inconsistency in designation of nomenclature groups in paleoanthropology are indicative of the lack of a generally accepted definition of the basic category of species.
The History of Zoological Classification
Typological synopsis of aristotle
It was Aristotle that gave the first scientific description of animals (more than 500 species) in his work «Historia animalium» written about 334-330 years B.C. [Aristotle’s 1878]. He introduced a comparative analysis method for differentiation and identification of animals. In his work, he employed the terms “genos” (genus) and “eidos” (kind) to categorize animals on the basis of their similarity and complexity (Aristotle’s 1878: Book 1). However, these terms are not phylogenetic categories but rather logical universals, also referred to as typological universals. Aristotle did not classify animals in the proper sense of the word, he rather grouped them into orders in the ascending order from simple to complex, thus anticipating the medieval concept of «scala naturae», the Ladder of Nature (Aristotle’s 1878: Book 5).
Besides, he assigned the man biologically to the animal group (Aristotle’s. 1878: Book 1) for physiological reasons (Aristotle’s. 1878: Book 2), and considered the man to be the most complex animal (Aristotle’s. 1878: Book 5), and in some translations – the most perfect one.
It must also be mentioned that Aristotle admitted of a possibility that some changes in animals through intercrossing (Aristotle’s. 1878. Book 8) or domestication (Aristotle’s. 1878: Book 1).
Notice: we used several translations in this research, but the one chosen (Aristotle’s 1878) had a convenient numeration of passages.
Genus-Species systematics of c. linnaeus
C. Linnaeus was the first to propose a scientific classification of animals in his “Systema Naturae” (1735), where he included the “Regnum Animale” kingdom in the corpus of nature «Corpore Naturalis» (referred to as “Imperium Naturae” in later editions). The Kingdom consisted of four subordinated hierarchical categories presented in the form of tables, where he put the first 584 species with description of their main characteristics.
Later C. Linnaeus presented the theoretical bases for the classification in his work “Philosophia botanica” (1751), though it was limited to plants only. This paper introduced the notion that later paved the way for the theory of evolution, “The plant variety changes under the influence of an accidental cause, climate, soil, temperature, wind, etc.” (Varietas est Planta mutata a causa accidentali, Climate, Solo, Calore, Ventis, &c) [Linnaeus 1751]. In order to correlate the original form of the plant with its present state, C. Linnaeus established two categories: “species” and “genera”. The term “species” is Latin for “image” and denotes the modern state of a plant, while “genera” means “origin” and includes the description of presumably inherent characteristics of the initial form.
For C. Linnaeus, the species and the genus were always the work of nature, but as far as there was no description of the initial forms, he proposed the “genus-species” axiom in order to restore the relations. The axiom establishes that only the structure of fructification organs can reach the present moment without changes, and therefore “there are as many genera as there are variations in reproductive organs in natural species” (Genera tot dicimus, quot similes constructae fructificationes proferunt diversae Species naturales”- lat.) [Linnaeus 1751: Canon 159]. Having established the category of species as the basic unit of his classification, C. Linnaeus repeated in several canons that “the primary division of plants (in classification) should be based on the parts of the fructification alone” (“Dispoditio vegetabilium primaria a sola fructificatione desumenda est”- lat.) [Linnaeus 1751]. This axiom determined the common genus of externally different species or species inhabiting different geo-climate zones.
C. Linnaeus underlined that “without knowledge of genus the knowledge of species cannot be valid” (Sine notitia Generis nulla certitude specie). He confirmed this genus-species relation by subject-attribute form of a group name, where the genus had a subject basis, and a species had either trivial or differentiating character [Linnaeus 1751].
The shift from a typological synopsis to a classification based on a relationship system was reflected in the term «Systema Naturae». Though C. Linnaeus did not establish a similar genus-species axiom for animals, he identified some properties for a classification on the order level.
Besides, in his “Observationes” (1735) C. Linnaeus was the first to formulate, though implicitly, two main characteristics of a species, namely reproductive isolation from closely related species and continuity of origin (Borkin 2009).
Ch.R. Darwin’s genealogical classification
By correlating the consistency of deposits with the increasing complexity of paleozoological findings of “organic beings”, Darwin dated back the emergence of original biological forms long before the Selurian Period (443-416 mya) and proposed the theory of Natural Selection (i.e. the theory of descent with modification) [Darwin 1859].
According to the theory, “organic beings” exposed to new geoclimatic environment undergo not only a genus–species change (according to C. Linnaeus) but also a series of deep morphological changes and branching. “For as all the species of the same group have descended from some one species, it is clear that as long as any species of the group have appeared in the long succession of ages, so long must its members have continuously existed, in order to have generated either new and modified or the same old and unmodified forms” [Darwin 1859]. It allowed [Ch.R. Darwin 1859] to put forward a new principle of classification, “The natural system is a genealogical arrangement as in pedigree tree, but with the grades of acquired difference marked by the terms varieties, genera, families, orders and classes”.
[Darwin 1859] attributed the arising diversity of the organic world to the divergence of characteristics in different areas due to “different conditions of life” and the following modification of individuals resulting in the formation of separate (phyletic) lines of descent and in the emergence of species different from the original. Darwin’s concept included both “horizontal” isolation from coexisting species and “vertical” delimitation of ancestral and descendant species along the phyletic line.
Darwin neither distinguished the level of modification of individuals on the phyletic line that leads to the formation of a new species, different from the original, nor gave it any definition. He considered the term “species as arbitrarily given for the sake of convenience to a set of individuals closely resembling each other” [Darwin 1859]. He also pointed out that “the amount of difference considered necessary to give to two forms the rank of species is quite indefinite” [Darwin 1859].
This concept is shared by most modern scholars, “Species are evolving systems, and the vertical delimitation of species in the time dimension should in theory be impossible” [Mayr 1969].
Paleoanthropological ICZN Classification Paleoanthropology is yet to come up with a generally accepted definition of species as a basic unit for classification of evolving groups of individuals.
For a species’ name to be registered in a ZooBank, its identification should be confirmed by a “name-bearing type” according to the ICZN Principle of typification (1999: Art.61). “Each nominal taxon in the family, genus or species groups has actually or potentially a name bearing type. The fixation of the name bearing type of a nominal taxon provides the objective standard of reference for the application of the name it bears” (ICZN 1999: Art.61.1.).
Though preamble of the code declares only the principles of registration for zoological groups, and rules of their identification are referred to the sphere of taxonomy, but in fact species assignment demands a typological diagnosis as “the name bearing type of a nominal species- group taxon is a specimen or a set of specimens” (ICZN 1999: Art.61.1.2.).
Only fossilized remains may serve as the name-bearing type in paleoanthropology, therefore researchers have to use all their metric measurements available to diagnose and differentiate a nominal species-group taxon (about 70 craniodental and about 40 postcranial ones recently). As a rule, it is the numerical taxonomy methods from the sphere of phenetics that are applied to compare this amount of characteristics. For this purpose, all the metric characteristics of an individual are assigned a priori equal “diagnostic weight”, then all these metrics are united in a so-called “operational taxonomic unit” (OTU) [Sneath & Sokal, 1973]. Pairwise comparison of the OTUs of the individual groups is instrumental in determining the similarity and dissimilarity levels and providing reference standards for name-bearing taxon fixation.
Thus, the assignment of A. sediba species was done on the basis of 29 metric characteristics of fossils different from those of A. africanus. In its turn, 16 metric characteristics distinguish A. africanus from A. afarensis [Berger Lee R. 2012].
However, metric values of bone remains belonging to the individuals of the group have deviations that demonstrate the Gaussian normal distribution, so even within one population of individuals there are different values for one and the same metric characteristic, which suggests different typological species of these individuals. As far as bones are exposed to erosion, and often are damaged by predators and scavengers, the arbitrary set of characteristics of the only fossil designated as holotype (ICZN 1999: Art.61) may set OTU nomenclature bases for species assignment which do not properly reflect the whole natural group, but only its part, i.e. a subspecies, a population or a cluster.
For example, type specimen OH 5 “Zinj” (1.8 mya) of A. boisei and ТМ 1517 (2.0-1.5 mya) of A. robustus typologically represent different species, are trophically indistinguishable, and occupy the common ecological niche due to their radicophagous diet [Vyrskiy 2017]. All these factors refer them to one biological species according to [E. Mayr 1969].
Apart from that, spatial proximity of fossils in a deposit does not indicate that they belong to the same species. For example, a mandible fragment U.W. 101-377 of the original species homo naledi [Berger et al. 2015] shows omnivorous specialization morphology with a significant proportion of meat, but the maxillary and mandibular teeth of the LES1 cranium from the collection of the same species [Hawks et al. 2017] suggest specialized herbivorous diet [Vyrskiy 2018], which is indicative of their trophical incompatibility and puts them into different biological species.
As it is impossible to compare the OTUs belonging to different parts of skeleton, this leads to a certain increase in the number of species.
The phyletic collisions may appear if two or more nominal genus-group taxa have the same type species (ICZN 1999: Art.61.3.3.).
Furthermore, numerical cluster analysis (in spite of the complexity of this method) shows a phenetical rather than phyletic relationship, i.e. some likelihood or affinity [Sneath & Sokal, 1973]. Therefore, the nomenclature assignments do not correlate to the real phylogeny of the groups under study, and it induces the founders of collections to specify the phylogenetic system alongside the nomenclature classification.
For example, for Au. sediba there is a five-level hierarchical classification together with four hypotheses concerning its phylogenetic position [Berger 2012]. Some researchers refuse complex statistic calculations entirely. O. tugenensis has been assigned a five-level hierarchical ICZN structure and an origin from Samburupithecus kiptalami species of Late Miocene from Kenya (9.5 mya) [Senut et al. 2001].
Taking into account that “the name bearing type of a nominal genus is a nominal species called the “type species” (ICZN 1999: Art.67.1.), and “the name bearing type of a nominal family-group taxon is a nominal genus called the “type genus” (ICZN 1999: Art.63.), then the assignment of a typological cluster to a species will lead to the artificial character of its genus and family, and inclusion of such species into the order Primates will cause hierarchical splitting of the whole classification.
Meanwhile, researchers are forced to make various artificial constructions, for example, to introduce the concepts of mosaic evolution, microevolution, parallel evolution, and consequently to describe ‘mosaic monsters’ having the hands of a homo and the legs of an ape, or vice versa, and to designate mosaic phylogenetic names, for example, a “homo-Like Australopith”. In some cases, when the fossils of later individuals demonstrate no diagnostic characteristics of an assigned species, which indicates their extinction, then a concept of “introgressive hybridization” is introduced. Having overcome this limitation, this concept allows the research of the further evolution of typologically extinct species [Hawks, 2017].
As Article 2 of the Main Decisions [ICZN 2008] states, “The requirement for registration in ZooBank of new scientific names in electronic works was changed to a requirement for registration of the work itself”. That makes cataloging a bit difficult and provides for the arbitrary treatment of earlier established names.
In fact, ICZN typification does not consider such characteristics of evolving groups assigned for species as succession and vertical delimitation from both ancestral and descendant species on the phyletic line. It becomes especially vivid in paleoanthropology as it contradicts the real natural groups, creates artificial splitting of classification and hinders the reconstruction of an equal phylogenetic system of species (Ch.R. Darwin’s genealogical system).
An Attempt to Define a Species in Paleoanthropology, its Diagnostics and Differentiation
Characteristics of evolving groups of individuals
Taking into account all species’ characteristics suggested by C. Linnaeus and supplemented by Ch.R. Darwin’s “theory of descent with modification” we may formulate a general definition of a species in the following way: a sequence of reproduced generations of individuals that are naturally separated from coexisting closely related species and delimitated both from ancestral and descendant species on the phyletic line.
Different branches of zoology apply various characteristics of individuals to differentiate the species, i.e. morphology, ethology, physiology, reproduction, genetics, etc. As a rule, groups of coexisting animals are compared.
Diagnostics and differentiation of evolving groups of paleontological individuals require the characteristics that would factor in not only distinctness of co-existing species, but delimitation of species on the same phyletic line. As there is no generally accepted rule of delimitation of species on the phyletic line, we denote a succession of “unbroken line of generations” as a Self-reproductive group (an r-group).
Fossils are the only source of data in paleoanthropology. Their metric measurements determine some functional characteristics of r-group individuals. In this research we use the term features to designate the metric measurements of fossils and their ratios. The term characters will be used to define the functional (integral) characteristics of individuals, such as locomotion or diet.
Reproductive Distance Method of Characters for Finding the Intervals with Unchangeable Adaptive State of Groups
In the course of evolution some characters of an r-group disappear, while some others emerge, but the features of the fossils only vary in size. The emergence of new functional characters means the change of adaptation of the r-group to external environment. The time interval on the phyletic line during which the set of characters remains unchanged shows the invariance of its environment adaptive ability.
It allows us to introduce the notion of an adaptive state of an r-group which is determined by a set of functional characters of individuals. The borders of an adaptive state of an evolving r- group are established by the emergence or disappearance of characters on the basis of features of the fossils. For this purpose, we assign a reproductive distance parameter to each character, which means a time-interval on the paleontological time scale during which the character is observed in deposits. It is reproduced by generations of individuals-their carriers.
In reality, individuals have a lot of characters which reflect not only the current adaptive state, but also some of their ancestors, acquired by the r-group on the phyletic line much earlier. If we allocate characters on the paleontological time scale in correlation with their reproductive distance, then the hierarchical system of characters will show interval succession, where each of them determines distinct adaptive state of the r-group.
Reconstruction of Adaptive States of Bipedal Primates in Period under Study
It is the “terrestrial bipedalism” character that possesses the greatest reproductive distance equal to the whole interval of the research (6.0-1.0 mya). We created an r-group with the phylogenetic title “bipedal primates” which embraces individuals of all original species from Table 1, including the early homo, as they have the same locomotion system.
The analysis of craniodental architectures of the individuals of this r-group suggests two diets: a herbivorous, amylum-full diet consisting of cereal grains, roots, bulbs of field and coastal herbs (a radicophagous diet); and an omnivorous diet, including a significant proportion of meat [Robinson 1954]. The difference between the two diets and teeth-jaw apparatus of the individuals is marked on the dichotomy level [Lucas and Peters 2000]. It allows us to distinguish two diet-incompatible adaptive states in the r-group of bipedal primates.
As far as their mutual systematic position is unknown, we distinguish them as separate r- groups of lower level and assign phylogenetic names of “radicophagous” and “omnivorous”. It should be noted that no other types of diet of bipedal primates have been recorded.
The characteristics distinguishing the adaptive states of radicophagous and omnivorous r- groups are given in Table 2.
MD/LL, ratio of the mesiodistal diameter to the labiolingual diameter;
CA, cortical area; MA, medullary area; TA, total periosteal area (TA = CA + MA);
%CA = (CA/TA) × 100;
Cerebral index, CI = ([0.91 × ECV)/Pb] - ECV)2
ECV – endocranial volume in cc; Pb is the body weight in g. Sources of data: [Vyrskiy 2017]
The comparison of observation intervals of the r-groups radicophagous and omnivorous by deposits and the location of deposits indicate their sympatric coexistence on the continent. Diet incompatibility of adaptive states of the r-groups demonstrates their distinct phyletic lines.
The area of the central Afar rift valley in Ethiopia and Kenya has been reported to provide the first evidence of the use of stones for the removal of flesh from ungulate carcasses ~3.3 mya [McPherron et al., 2010], and the first collection of stone tools with sharp edges obtained by splitting and crushing stones [Harmand et al., 2015]. The 2.6 mya deposits in this area show the first “true” stone tools, produced by manufacturing techniques of the Oldowan industry [Semaw, 2000; Braun et al., 2019].
Active instrumental exploration of the environment reflects a new adaptive state of individuals and points to the emergence of one more r-group 2.6 mya. As far as the only purpose of flesh removal was meat, it may be concluded that some meat-eating population close to the omnivorous r-group acquired the skill of making stone tools. While registering original bipedal primates related to “stone tools” artefacts, the scholars refer them to the homo genus, thus allowing us to give the phylogenetic name “homo” to a new r-group.
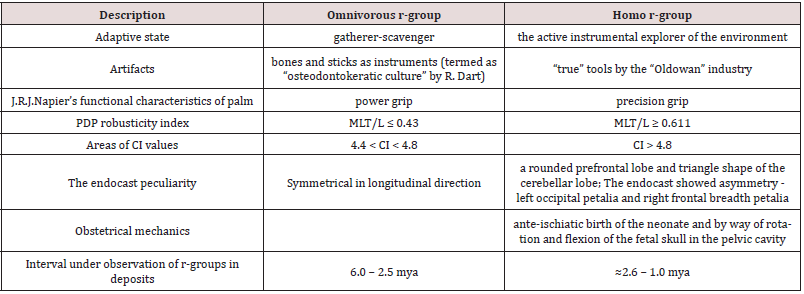
Table 3 shows the characteristics distinguishing the adaptive states of the r-groups omnivorous and homo.
MLT, mediolateral width at the pollical tuft; PDP - the pollical distal phalanx; L, length of the PDP.
Sources of data: [Vyrskiy 2017].
The comparison of observation intervals of the adaptive states of omnivorous and homo r- groups and their similar diet adaptation presumably prove that they belong to one phyletic line, delimitated by the emergence of the functional character of “true” Oldowan tools.
As a result of distribution of characters on the paleontological time scale according to their reproductive distance, we see three adaptive states and corresponding three r-groups of bipedal primates: radicophagous (5.8 – 1.34 mya), omnivorous (6.0 – 2.5 mya), and homo (2.6 – 1.0 mya) in the observed deposit interval. However, the applied method fails to show their phylogenetic relations.
Reconstruction of phyletic relations between adaptive states by the method of continuity of adaptive lines of features
The Supporting Information hereto include Tables 4, 5, 6 (Vyrskiy 2017: Tables 5, 6a, 6b) which represent features “individual’s body weight” (Pb) and “cerebral index” (CI) for individuals of radicophagous, omnivorous and homo r-groups from the observed deposit interval.
Figure 1 presents the Pb data on the paleontological time scale.
Figure 1: Changes in the body weights Pb of individuals belonging to the radicophagous, omnivorous and homo r-groups. Sources of data: Tables 1, 2, 3 (SI). Note: For some intervals, an average of the values from Tables 1, 2, 3 (SI) is given
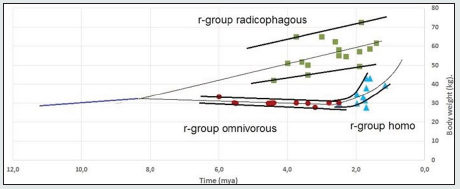
The reproductive succession of generations implies an unbroken hereditary transmission of features which only change their values when r-groups shift to new adaptive states. However, due to incompleteness of paleontological data, there are interruptions on Figure 1 that demand interpolation of data on the paleontological time scale to establish reproductive relations between r-group’s adaptive states.
We limited the range of values for each r-group (Fig.1) by upper and lower envelopes which show certain deviation of Pb values for each time point. Morphological measurements are known to show a normal Gaussian distribution. Consequently, the deviation of Pb in Figure 1 corresponds to the interval width (Pb -3σ; Pb +3σ) (Fig.2) which embraces 99,72% of all individuals (σ - Mean square deviation).
Figure 2: Single-step normal Gaussian distribution of the feature single values among the r- group individuals.
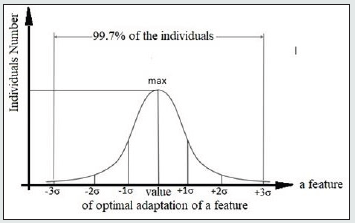
The point on the x-axis that corresponds to the largest number of individuals on the y-axis marks out the optimal adaptation point of the r-group individuals to the environment. Given the symmetric nature of the Gaussian distribution function (Fig.2), the points of optimal adaptation of a feature will be located on the median lines of r-group values. Actually, median lines restore the lack of continuity caused by incomplete data, and are the lines of optimal adaptation.
We plotted the median, or adaptive lines, for the range of Pb values on Figure 1: as linear regression for the radicophagous and omnivorous r-groups, and as sedate regression for the homo r-group.
As we can see from the graph:
a) Adaptation line Pb of the r-group radicophagous crosses neither the omnivorous r-group line, nor the homo line, and their ranges do not overlap, which supports the idea of reproductive incompatibility of the radicophagous-omnivorous and radicophagous-homo sympatric pairs.
b) In the interval of 2.6-2.5 mya we observe a merge of adaptation lines Pb omnivorous and homo, which suggests reproductive succession in the contact point and the consolidation of two adaptation lines into one evolutionally unbroken line. It means a direct reproductive relationship and hierarchical ranging of the omnivorous (maternal) and homo (daughter) r-groups.
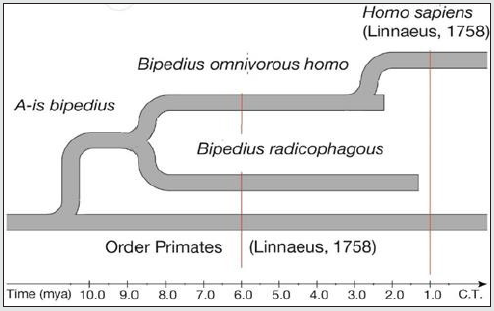
c) When extrapolating adaptation lines of the r-groups radicophagous and omnivorous beyond the observed intervals, they will demonstrate merging in the interval of 9.0-8.0 mya, pointing at the possible origin through branching of a maternal r-group that featured bipedalism. This hypothetical maternal r-group has been assigned a phylogenetic name “bipedius”.
d) When extrapolating adaptation lines Pb of the r-groups radicophagous and homo, they show possible crossing and merging of their ranges in the interval of 1.0-0.5 mya.
Figure 3: Cerebral index (CI) changes in the r-groups radicophagous, omnivorous and homo (Tables 1, 2, 3 SI).
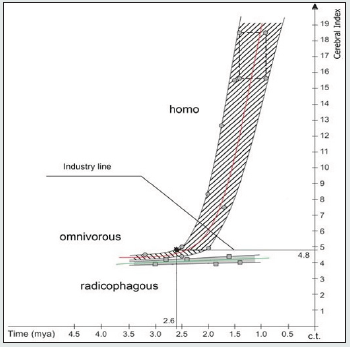
Figure 3 confirms the following conclusions obtained on the basis Figure 1:
a) Adaptation lines CI of the sympatric r-groups radicophagous and omnivorous do not cross, but they show asymptotic type of CI closeness in deposits older than 3.5 mya, which indirectly indicates their common origin from a maternal bipedius r-group Figure 1.
b) Extrapolation of CI adaptation lines of the r-groups radicophagous and homo in 1.0-0.5 mya excludes their possible merging, which proves their mutual reproductive distinctness.
c) Adaptive CI lines of the r-groups omnivorous and homo in the interval of 2.6-2.5 mya show a merge resembling Pb in Figure 1, thus proving succession, direct reproductive relationship and hierarchical “maternal – daughter” ranking of their adaptive states. d) From 2.6 mya, the homo r-group indicates exponential growth of CI values in Figure 3, pointing at extreme level of hominization, and the beginning of this growth correlates with the time of “true” Oldowan tools (2.6 mya). The 2.6 mya point on the upper envelope adaptive line “omnivorous – homo” indicates CI=4.8 on the y-axis. We named CI=4.8 on the y-axis the “industry line” and established the marginal value of feature, thus distinguishing the maternal omnivorous r-group (CI < 4.8) from the affiliated homo r-group (CI ˃ 4.8).
Adaptive species axiom in paleoanthropology
The analysis enabled us to distinguish an r-group of evolving individuals as a naturally distinct entity of individuals whose adaptive state determined by a set of functional characters remains unchanged during some time. Each adaptive state meets the species’ characteristics in terms of Linnaeus-Darwin and may be regarded as a basic unit for the phylogenetic classification used to designate the category of species.
As the functional characters of individuals are identified by fossils’ metric features, the designation of a category has exclusively instrumental nature, and must be suggested as an axiom. In order to distinguish the suggested species category from the generally accepted meaning of the term “species”, assigned in accordance with the ICZN typification, we supplement it with an attribute ‘adaptive’, bearing in mind that the basis for differentiation is the adaptive state of an r-group.
The axiom of adaptive species in paleoanthropology, “Reproductive generations of individuals whose adaptive state, determined by a set of functional characters, remains unchanged for some time”.
We applied the given axiom of adaptive species to phyletic systematization of African bipedal primates.
Phylogenetic System of Adaptive Species of African Bipedal Primates
a) Presumably, a population of some arboreal species from the order Primates [Linnaeus 1758] became bipedal and formed a hypothetical r-group bipedius. Taking into account a newly formed adaptive state of the r-group, and for the purposes of theoretical systematization and further study, it is necessary to assign an adaptive species category and the name of A-is bipedius to the r-group bipedius. The attributive part of the name of the species reflects the main functional adaptation. Neither the region, nor the time of species emergence were studied in the frames of the present research, the diet is also unknown.
Figure 1 demonstrated the division of this species into two affiliated diet-incompatible r-groups radicophagous and omnivorous in the time interval of 9-8 mya.
b) Presumably, the r-group radicophagous emerged about 9-8 mya as a result of branching of the maternal A-is bipedius species. The adaptation state is represented by terrestrial amylumbased diet. Table 2 presents a virtual image of an individual of the said r-group.
The earliest samples of individuals belonging to the r-group radicophagous were identified by several teeth [Vyrskiy 2017] from the original Ardipithecus (ramidus) kadabbа species collection from 5.8-5.2 mya deposits. The latest samples of fossils were described in OH 80 [Dominguez-Rodrigo et al., 2013] from the deposits 1.34 mya, thus we may assume further extinction of the r-group on the African continent.
The r-group radicophagous satisfies the adaptive species axiom definition in the interval ≈ 9 – 1.34 mya and we may assign the category of adaptive species to this r-group and name it bipedius radicophagous. The subject name identifies the maternal diagnosis, and the attributive name shows the differentiating diagnosis, i.e. diet specialization.
The African continent is the areal for species of the present research. The hypodigm of the species is represented in Table 4(SI).
c) The r-group omnivorous, as well as the radicophagous presumably originated about 9 – 8 mya through branching of the A-is bipedius. The adaptive state is presented by terrestrial omnivorous diet of gatherers-scavengers (“osteodontokeratic culture” by R. Dart). The virtual image of an r-group individual is presented in Table 2; 3. The first samples of a terrestrial omnivorous individual are found in the collection of the original species O. tugenensis [Senut et al. 2001] from the deposits 6.2–5.65 mya.
A population of the r-group omnivorous which inhabited the region of the Middle Awash river in Ethiopia and Kenia in 2.6 mya began to produce “true” Oldowan tools and formed a new affiliated adaptive state [Braun et al., 2019; Semaw, 2000]. Through the next 100 thousand years the r-group omnivorous became extinct and was substituted by the affiliated r-group homo on the continent. The latest individuals of the r-group omnivorous were excavated in the deposits 2.5 mya in Sterkfontein and Taung on the territory of South Africa.
Table 2 and Figures 1 and 3 show a long sympatric coexistence of the r-groups omnivorous and radicophagous on the continent. The data also confirm their reproductive incompatibility.
The r-group omnivorous meets the axiom definition in the interval ≈ 9-2.5 mya, which allows us to assign an adaptive species category and the name bipedius omnivorous to this r-group. The subject name shows the maternal diagnosis, while the attributive one denotes the differentiating trophic character.
The hypodigm is presented in Table 5 (SI). Within the frames of the current article, the African continent was the areal of this species.
d) The r-group homo branched approximately 2.6 mya off a B. omnivorous species population, the individuals of which began making the first “true” Oldowan stone tools [Braun et al., 2019; Semaw, 2000]. Having an adaptive advantage, the r-group homo ousted the maternal species from the continent by 2.5 mya.
The adaptive state of the r-group is determined by the industrial usage of natural resources-search and explorations of specific types of stones, necessary for the manufacture and instrumental use of the environment. This fundamental difference between the adaptive state of the r-group homo and that of the maternal B. omnivorous allows us to assign an adaptive species category to this r-group. Its adaptive state coincides entirely with that of the homo sapiens [Linnaeus, 1758]. Besides, in spite the dramatic difference between the first stone tools of the r- group and the modern ones, the industrial nature of economic activity and its natural source have hardly changed until the present moment.
We believe that a new species should be assigned the name homo industrial, but as the virtual morphometric image of the r-group homo individuals described in Table 3 does not contradict but supplements the diagnostic and differentiation characteristics mentioned by Linnaeus for homo sapiens (1758; 20-23pp), it might be concluded that the r-group homo of t he species level and the H. sapiens species are the same adaptive species.
Given the dynamics of Pb and CI changes within the observed period and the evolution of industrial character of the economic activity until now, one may state the evolutionary intermediacy of H. sapiens formation by E. Mayr.
So, the adaptive H. sapiens species appeared in the basin region of the Middle Awash river in Ethiopia and Kenya 2.6 mya and exists today.
The list of individuals from the African deposits 2.6-1.0 mya is represented in Table 6.
e) We added the second attribute homo to the name of B. omnivorous to restore the reproductive relations between H. sapiens and the maternal B. omnivorous species. Besides, the attribute homo underlines the fact that the B.o. homo species is different by its adaptive and morphological nature from all apes and is not an intermediate species, as far as it originated from the maternal species which already possessed terrestrial bipedalism.
The hand morphology of B.o. homo also supports this assumption because it demonstrates an ability to use improvised objects (horns, stones) in daily activities. Such skills could emerge only with the formation of a somatic ability to repeat an useful precedent, for example the use of horns and hoofs by animals while attacking or defending.
f) The important peculiarity of H. sapiens emergence is that it appeared not as an adaptation to the changing environment but as a result of emergence of the maternal population of individuals which possessed an inducing capability to invent useful precedents for further imitation and use by other individuals. These precedents included the search of specific deposits, manufacturing technologies, and use of stone tools.
The scope of this research does not cover the study of somatic characteristics of individuals creating the industrial niche of H. sapiens. However, it is also believed to be necessary to point at a certain peculiarity of thinking of some individuals which is reported in the described stone artifact collection about ≈3.3 mya [Harmand et al., 2015]. The individual tried out all types of rock and all sizes of stone billets available to get the sharp edge best suited to remove the flesh, and therefore acquired experience.
g) The reproductive relationship system of the adaptive species of bipedal primates allows us to unite them into a system, and to map a phylogenetic scheme on the paleontological time scale.
Methods and Results
The present paper formulates the adaptive species axiom for paleoanthropological groups of individuals as “Reproductive generations of individuals whose adaptive state, determined by a set of functional characters, remains unchanged for some time”. The unchanged adaptive state of a species allows us to delimitate it from ancestral and descendant species on its own phyletic line and separate it from simultaneously coexisting closely related species that satisfy the species criteria suggested by Linnaeus and supplemented by Ch. R. Darwin’s “theory of descent with modification”. A method was suggested to distinguish the intervals of r-group adaptive states on the phyletic line. It consists in assigning a “reproductive distance” parameter to each functional character. This parameter constitutes a time-interval on the paleontological time scale during which the given character is observed in deposits, i.e. reproduced by generations of individuals-their carriers. The distribution of characters along the paleontological time scale in accordance with their reproductive distance shows a hierarchical system of characters and divides a phyletic line into successive intervals, each of them determining a certain distinct adaptive state of the r-group, described here as an adaptive species. A method of adaptive lines was applied to differentiate between simultaneously coexisting closely related species with possible coincidence of some features. The method is based on a peculiarity of reproduction of a metric feature whose value forms an unbroken succession when inherited. It may be presented graphically as a function on a paleontological time scale and studied mathematically. This function was given the name of an adaptive line. Comparing the sets of homologous features’ adaptive lines of sympatrically coexisting groups of individuals enables us to separate phyletic lines of closely related species.
The reconstruction of adaptive states of African bipedal primates from 6.0-1.0 mya deposits, performed on the basis of proposed methods showed sympatric coexistence of two closely related adaptive species, which were assigned the names of bipedius radicophagous and bipedius omnivorous homo. These species originated 9-8 mya by branching off the maternal species A-is bipedius which already possessed bipedalism, and they became extinct 1.34 and 2.5 mya correspondingly. However, one of the B.o. homo populations, inhabiting the region of the Middle Awash river in Ethiopia and Kenya, formed the affiliated species 2.6 mya with the type of industrial activity and its natural source equivalent to H. sapiens. The comparison of the key characteristics of the virtual image of a new species (Table 3) with the diagnostic and differentiating characteristics of H. sapiens [Linnaeus 1758] showed their identity and enabled us to suggest that this is the same species which emerged 2.6 mya and still exists today.
Discussion
This paper does not consider the correlation between the suggested phylogenetic scheme and the original species of African bipedal primates of the observed interval. The main purpose of this article is to suggest instrumental methods of reconstruction of a phyletic relations system for evolving groups of individuals that would satisfy Ch. R. Darwin’s genealogical principle. A new adaptive species category proposed herein has primarily theoretical nature. This category is outside the ICZN typology and was introduced for the sake of systematization and further study of phylogeny. The research is limited by the lack of African paleoanthropological material, as well as by conventional nature of calculations of values and ratios of fossils’ features. The results obtained by new methods of fossils research, such as isotopic evidence of dietary variability, were outside the scope of the present paper. Neither have we used the collections of bipedal primates of the European and Asian continents.
Conclusion
a) The proposed adaptive species axiom and methods of its diagnostics and differentiation allowed us to reconstruct the phyletic system of evolving groups of bipedal primates that satisfy the requirements of Ch. R. Darwin’s genealogical principle, and also to systematize almost all significant collection samples in the period of study. b) The research of 9-8 mya deposits is of immense importance as it is the interval when presumably two diet incompatible adaptive species with a similar locomotor system diverged. c) The study of evolutionary intermediacy of homo sapiens formation to the present day is of no less importance. It may be observed through an exponential growth in the number of individuals possessing specific creative thinking who produce an increasing amount of increasingly complex useful precedents for industrial imitation.
References
- Then took they a chariot and two men, the best of their people, sons twain of Merops of Percote, that was above all men skilled in prophesying, and would not suffer his sons to go into war, the bane of men; but the twain would in no wise hearken to him, for the fates of black death were leading them on. These did the son of Tydeus, Diomedes, famed for his spear, rob of spirit and of life, and took from them their goodly battle-gear (Il. XI, 328-334, transl. by A.T. Murray, revised by W.F. Wyatt, Cambridge [MA], Harvard University Press, 1924 [I. Books I-II], 1925 [II. Books XIII-XXIV], in all Iliadic quotes).
- P. Vidal-Naquet, in J.-L. Backès (éd.), Homère, Iliade, Paris, Gallimard, 2013, p. 592. The literature on this subject is conspicuous. Here it is enough to remember that the expression meropes anthrōpoi is one of the formulas of the geographical catalog of the Hymn to Apollo (H. Koller, Πόλις μερόπων ἀνθρώπων, «Glotta», 46, 1968, pp. 18-26), while R. Arena, Per una interpretazione dei termini Meropes e Chaoi, «Rendiconti. Istituto Lombardo, Accademia di Scienze e Lettere, Classe di Lettere, Scienze morali e storiche», 108, 1974, pp. 417-437, dwells on the etymology of the Meropes, interpreted as «those who were born from the earth». It should also be remembered that Merops was the king of Cos, native and eponymous, and the island itself bore the name of his daughter: cfr. Steph. Byz. s.v. Κῶς (p. 402 s. Meineke); Pind. Nem. IV 42, Isthm.VI 41-45; Plut. Quaest. gr. 58; Hsch. s.v. Μέροπες, μ 865 Schmidt. Cfr. also P. Ramat, Nuove prospettive per la soluzione del problema dei Mέροπες di Cos, «Atti e Memorie dell’Accademia Toscana di Scienze e Lettere “La Colombaria”», 24, 1959-1960, pp. 131-157, and M. Lejeune, Discussions étymologiques, «Revue des études Anciennes», 63, 1961, pp. 433-438. V. Pisani, Mέροπες ἄνθρωποι, «Acme», 29, 1976, pp. 5-7, identified in the attribute designating the predoric population of Cos as well as the genus of birds that usually lay their eggs on the ground, a synonym of chthonios («linked to the earth»). Among those who have deepened the phonetic aspect, with the related etymological implications, there have been A. Quattordio Moreschini, Le formazioni greche suffissate in -op-, -ōp-: μέροπες ἄνθρωποι e ἑλίκωπες Ἀχαιοί, «Studi e Saggi Linguistici», 21, 1981, pp. 41-77, M. Morani, Tre aggettivi omerici, «Sileno», 16, 1990, pp. 151-160, and A. Annus, Are there Greek rephaim? On the etymology of Greek meropes and titanes, «Ugarit-Forschungen. Internationales Jahrbuch für die Altertumskunde Syrien», 31, 1999, pp. 13-30, who has caught original correspondences with Semitic roots. The interpretation of B. Pastor de Arozena is completely different, as well as that, more recent, of A. Willi. The first scholar argues that the word meropes derives from the Indo-European root *mer, which in Homer and Hesiod expresses brilliance (B. Pastor de Arozena, Meropes «glitterisch», «Classical Philology», 88, 1993, pp. 137-138). The second one, on the other hand, evaluates the expression meropōn anthrōpōn as the evolution for metric reasons of an older *meiropōn anthrōpōn, which would belong to a phase of epic poetry prior to the final prosodic definition of the Homeric hexameter. The radical, *(s)mer, would be connected with μέρος, «part», and specifically would indicate the *meirops, or the «young member of the army who receives part of the military booty»: cfr. A. Willi, Mέροπες ἄνθρωποι, in N.N. Kazansky (ed.), Indo-European linguisticas and Classical philology. Proceedings of the 17th conference in memory of Professor Joseph M. Tronsky (June 24-26, 2013), Sankt-Peterburg, Nauka, 2013, pp. 893-894.
- From these by the casting of lots was I chosen to fare hitherward» (Il. XXIV, 400).
- XX, 49-51 (transl. by A.T. Murray, revised by G.E. Dimock, Cambridge [MA], Harvard University Press Harvard University Press 1919, just like in the next cases).
- Obstinate one, many a man puts his trust even in a weaker friend than I am, one that is mortal, and knows not such wisdom as mine» (Od. XX, 45-46).
- XVIII, 342.
- I, 250-252.
- http://lacampagnappenaieri.blogspot.it/2011/03/la-leva-obbligatoria-dellitalia-unita.html (link consulted on 22.7.2021).
- And fourth Antilochus made ready his fair-maned horses, he the peerless son of Nestor, the king high of heart, the son of Neleus; and bred at Pylos were the swift-footed horses that drew his car. And his father drew nigh and gave counsel to him for his profit, a wise man to one that himself had knowledge» (Il. XXIII, 301-305).

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...