Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1403
Case Report(ISSN: 2644-1403) 
Type I Citrullinemia: Use of the Ani Monitor (Analgesia and Nociception Index) for the Anesthetic Management of a Pediatric Patient with Type I Citrullinemia Volume 3 - Issue 5
Mercedes Benítez Jiménez1*, Ernesto Martinez2, Lola Pato2, María Angeles Rodríguez Navarro1 and Jose Antonio Castillo Bustos1
- 1Department of Anesthesiology, JM Morales Meseguer University, Spain
- 2Hospital General universitario Niño Jesús, Madrid, Spain
Received: November 25, 2020; Published: December 21, 2020
Corresponding author: Mercedes Benítez Jiménez, Department of Anesthesiology, Resuscitation and Pain Therapeutics, General Hospital, JM Morales Meseguer University, Spain
DOI: 10.32474/GJAPM.2020.03.000176
Introduction
Citrullinemia is an autosomal recessive genetic condition that falls under the spectrum of urea cycle disorders. The defective genes reduce the activity of enzymes needed to process nitrogen effectively. Two types exist: type I (neonatal onset) occurs when the defective arginosuccinate synthase enzyme fails to complete the third step of the urea cycle by converting citrulline and aspartate to L-argininosuccinate. Type II (adult onset) develops with the production of malfunctioning citrine proteins, which serve to transport molecules used in the metabolism of simple sugars, the assembly of protein, and the urea cycle [1]. In both types, the pathogenesis involves the build-up of ammonia, which results in neurologic symptoms, including lethargy, ataxia, and seizures. Hyperammonemia, which is considered for ammonium figures above 150 micromoles/L, is a medical emergency. It can cause life-threatening neurological symptoms and a delay in global psychomotor development. Therapeutic measures are aimed at reducing blood ammonium, for this should be discontinued the proteins of the diet, avoid the endogenous catabolism of these by giving caloric in glucose solutions, administering arginine, sodium benzoate and sodium phenylbutyrate; if unchecked, hemodialysis, haemofiltration or peritoneal dialysis should be instituted. Perioperative management of ammonia plays a crucial role in managing these symptoms and facilitating postoperative recovery [2].
Very little is known about the anesthetic management of this disease, in the literature there are only six cases described and the reports refer to a late anesthetic recovery with a prolonged anesthetic awakening, possibly in relation to elevated levels ammonium in the blood and/or with the choice of intraoperative anesthetics that included narcotics. On the other hand, monitoring of intraoperative analgesia in paediatric patients through the interpretation of heart rate variability with the ANI monitor (Analgesia Nociception Index®, Metrodoloris, Lille, France) will enable the postoperative pain prediction, will allow drug delivery to be headlined and block the response to the nociceptive stimulus. In the case we present, the anesthetic management was aimed at avoiding intraoperative hyperammonemia, in addition we perform a general anesthesia balanced with multimodal analgesia, we use short-life drugs with low accumulation rate. With these measures the objective was to avoid prolonged awakening and achieve an early extubation. As in other cases described, we use dexmedetomidine in continuous infusion during the procedure as it reduces the release of pain neurotransmitters by activating the receptors in the primary sensory nerves, which decreases general narcotic requirements. It also reduces sympathetic momentum and release of norepinephrine, which plays a crucial role in reducing nitrogen losses and converting proteins into ammonia [1].
Clinical Case
The case we handled was that of a girl of 14 years and 44 kg of weight, as a personal background highlighted: allergy to beta-lactams, type I citrulline with difficult control ammonia, spastic-dytonic tetraparesis of difficult management, intervened at 2 years of age for Nisssen gastrostomy due to neurogenic dysphagia, presenting in the intraoperative a cardiorespiratory arrest (PCR) secondary to hyperammonemia, severe psychomotor delay due to anoxic encephalopathy secondary to PCR and secondary epilepsy. In treatment with sodium phenylbutyrate (2g every 6 hours, 180 mg/kg/day), arginine (3.5g 4 times daily, 310 mg/kg/day), tizanidine (6 mg every 8 hours, 18 mg/day), oral baclofen (20 mg every 8 hours, 60 mg/day), trihexiphenidine (3 mg every 8 hours , 9 mg/day), levetiracetam (1000 mg every 12 hours, 2g/day), lamotrigine (150 mg every 12 hours, 300 mg/day), perampanel (10 mg every 24 hours), seretide (25/125mg 1 puff every 12 hours in chamber), melatonin (5 mg every 24 hours), variance (2-3 drops every 24 hours) , movicol (1 adult sachet per day). It was accepted for the placement of an intrathecal baclofen pump for difficult-tohandle spastic tetraparesis. The patient entered the night before the surgery, an ammonia control was performed elevated (60 mmol / L, normal between 9-33 mmol / L) and together with the Nutrition Service of our center was pauted hydration with glucose serum to 10%, L-Arginine and oral phenylbutyrate were administered 3 hours before the intervention with the aim of decreasing ammonium levels, oral baclofen was also partied before the intervention.
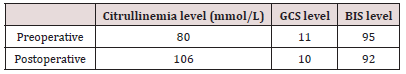
Upon arrival in the operating room, she was unreactive, drowsy, with a GCS (Glasgow Coma Score) of 11 (eye opening: 2, best motor response: 5, verbal response: 4) and BIS (Biespectral Index Score® Medtronnic, Minessota, US) in 95. The ammonium levels in the morning of the intervention were 80 mmol/L. The maintenance glucose serum infusion was continued during the intervention (110 ml/h) and a line of 0.9% physiological saline was added for bowling. Pulse oximetry, electrocardiogram, noninvasive blood pressure, BIS, TOF (Train of Four®, General Electric) and ANI were monitored. Esophageal temperature probe was used to monitor body temperature, to prevent hypothermia thermal blanket was placed (3M Bair HuggerTM Normotermia System, St. Paul, U.S.A) adjusting the temperature of the body to maintain a body temperature in around 36 ºC. We also indicate urinary cathetering for diuresis control. Propofol 2 mg/kg and rocuronium 1.2 mg/kg were used in the induction, antibiotic prophylaxis was performed with vancomycin 20 mg/kg and tobramycin 3.5 mg/ kg and levetiracetam 1mg iv was administered as anticonvulsant prophylaxis.
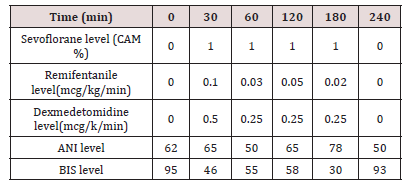
Orotracheal intubation (IOT) was performed by direct laryngoscopy using Macintosh laryngoscope with nº 3 blade, orthocheal tube (TOT) nº 7, flexometallic with cuffed and fastener, once the glottis was crossed the fastener was removed and the TOT was secured. Anesthetic maintenance was done with sevoflurane (1%), remifentanyl (0.02-0.1 mcg/kg/min) and dexmetomidine (0.25-0.5 mcg/kg/min) in continuous infusion. The dose of sevoflorane was adjusted according to BIS, to obtain figures between 40-60; the dose of remifentanyl and dexmedetomidine were adjusted according to the ANI for ranges between 50-70. Before the end of the intervention, we administer acetaminophen 15 mg/kg. The surgery lasted 4 hours, the dose of drugs used for anesthetic maintenance was reduced as the end of the procedure approached, so that when the patient had finished the spontaneous ventilation, the ANI was around 50-70 and we expect to have a similar BIS to the basal for extubation. After extubation, the patient maintained a good ventilatory mechanics and a neurological condition was like the previous one, with ammonium levels of 106 mmol / L. She was transferred to the intensive care unit, where she continued to extub and with good ventilatory function, saturating 96% to ambient air, hemodynamically stable, with good pain control without requiring rescue opioids and with a neurological examination like baseline (Tables 1 & 2).
Discussion
Monitoring of nociceptive responses caused by surgical stress has become relevant in recent times. The systemic response to surgical stress has postoperative repercussions, such as late recovery and worse pain management, longer stay in resuscitation and hospital units, and increased cost of health services. In contrast, treatment with higher doses of opiates than necessary may involve prolonged awakening situations and other side effects [3]. To optimize analgesia and avoid the use of opioids that could precipitate a prolonged awakening, in the case we present, we monitor the intraoperative analgesic level with ANI. The ANI monitors nociception based on the response of the autonomic nervous system to surgical aggression at the level of heart rate variability. In a recent prospective observational study investigated whether intraoperative changes in the high-frequency HR variability index correlate with clinically relevant nociceptive stimulation and the addition of analgesics, they could check that changes in the highfrequency HR variability index reflect alterations in the balance between nociception and analgesia and concluded that this index might be used intraoperatively to titrate analgesia for individual patients [4]. The algorithm of ANI converts the nociception, who depends on of the heart rate variability, to an absolute value between 0 and 100. Where an absolute value of zero corresponds to a maximum nociception and therefore to an aggressive sympathetic activity. While a value of 100 corresponds to a maximum analgesia and therefore to a maximum parasympathetic activity. Between 50 and 70 corresponds to an area of optimal anesthesia. In conclusion, monitoring of intraoperative analgesia in paediatric patients through interpretation of heart rate variability with the ANI monitor will allow us to predict hemodynamic response and its etiology. This monitoring of nociception will enable the titration of drugs in the intraoperative to block the response to the nociceptive stimulus and predict postoperative pain [5]. Monitoring of the ANI allowed to adjust the dose of drugs during the intraoperative, reducing the use of opioids to a low dose remifentanyl infusion, which proved decisive to achieve an early extubation and avoid prolonged awakening. The use of dexmedetomidine is especially beneficial in this patient because its sympaticolytical effect leads to a decrease in ammonium production and it also helped to decrease the dose of the other anesthetic and analgesic drugs (Figure 1).
When providing anesthesia in a patient with citrullinemia, effective management of ammonia levels perioperatively and intraoperatively may prevent delayed awakening by avoiding the neurological sequela of the disease process. Production of ammonia is augmented with accelerated protein breakdown during stress responses from stimuli such as surgery. A defective urea cycle leads to an accumulation of toxic byproducts during these catabolic states. The goal of anesthesia should be to optimize the patient’s ammonia levels preoperatively with treatments, such as phenylacetate and benzoate, and to select anesthetics based on their ability to prevent elevations in catecholamine and cortisol concentrations, in turn, preventing hyperammonemia intraoperatively. Our management of the patient before surgery involved glucose supplementation and sufficient hydration to prevent a catabolic state. Appropriate intravenous fluid resuscitation and maintenance help preserve anabolic physiology. Adequate caloric supplementation with carbohydrates and protein restriction also improves the anabolic state while optimizing utilization of energy [1]. Intraoperatively, the action of dexmedetomidine on α-2 receptors may have played a role in preventing hyperammonemia by reducing the overall stress response in our patient. Brede et al demonstrate the presynaptic feedback inhibition of noradrenaline via activation of α-2a receptors as well as the feedback inhibition of adrenal catecholamine release by α-2c receptors in studies performed on mice. In addition, the use of propofol as an induction agent and sevoflurane for maintenance may have had similar sympatholytic effects [1].
Igarashi et al. used opioid sparing. In our case, same as then, we were able to avoid opioid reversal by using the opioid sparing and analgesic effects of dexmedetomidine, which may have played an important role in managing pain while avoiding a prolonged emergence. Choice of paralytic and appropriate reversal may also influence awakening. Gharavifard et al suggest using shortacting and minimally metabolized drugs intraoperatively [6]. We use rocuronium at a dose of 1.2 mg / kg due to the suspicion of a possibly difficult airway. During the intervention it was necessary to administer an extra dose of 0.5 mg / kg relaxant. In our case we had sugammadex to reverse the effect of rocuronium although its administration was not necessary because when the surgery was finished the patient presented a TOF > 0.9.
Conclusion
Citrullinemia is a rare disease, with very few cases described in the literature and whose anesthetic management is not defined. Published cases appear to coincide with patients with type I citrullinemia more likely to have a prolonged awakening, hence the importance in controlling ammonium figures and administered drugs, which may trigger or aggravate this situation. The use of basic intraoperative monitoring and analgesia level, through ANI, improved the quality of anesthesiological care and met patient safety standards. We were able to optimize the dose of drugs administered, which contributed to the early extubation of the patient. Comprehensive intraoperative monitoring using BIS and ANI can be very useful in these cases where patients have severe psychomotor impairment that can hinder clinical assessment of the level of consciousness and pain. One of the goals of anesthetic practice is the control of surgical stress, being fundamental in our patient. The use of monitors such as ANI and the use of drugs such as remifentanyl, which provides effective analgesia and a rapid effect of action with fast elimination rate can contribute to this; and dexmedetomidine since in addition to the property of 2-agonist which allows centrally mediated anesthesia in the form of sedation, anxiolysis, analgesia and hypnosis, it could avoid an additional increase in ammonium figures thanks to its sympatholytic activity.
References
- Patel H, Kim J, Huncke TK (2016) General anesthesia in a patient with citrullinemia using Precedex as an adjunct to prevent delayed emergence. J Clin Anesth 33: 403–405.
- Igarashi M, Kawana S, Iwasaki H, Namiki A (1995) Anesthetic management for a patient with citrullinemia and liver cirrhosis. Masui 44(1): 96-99.
- Abad-Gurumeta A, Ripollés-Melchor J, Casans-Francés R, Calvo-Vecino JM (2017) Monitorización de la nocicepción, ¿realidad o ficción? Rev Esp Anestesiol Reanim 64(7): 406-414.
- Anderson TA, Segaran JR, Toda C, Sabouri AS, De Jonckheere J (2019) High-Frequency Heart Rate Variability Index: A Prospective, Observational Trial Assessing Utility as a Marker for the Balance Between Analgesia and Nociception Under General Anesthesia Anesth Anal 130(4): 1045-1053.
- Martínez-García E (2017) Monitoreo y tratamiento del dolor agudo en pediatrí Martínez-García. Rev mex antes 40(1): 285-286.
- Gharavifard M, Sabzevari A, Eslami R (2014) Anesthetic Management in a Child with Citrullinemia: A Case Repor Anesth Pain Med 4(3): e21791.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...