Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1403
Research Article(ISSN: 2644-1403) 
Effects of Low-Dose Transdermal Buprenorphine on Chronic Non-Cancer Pain, Functional Capacities, Behavioural and Cognitive Levels in Elderly Patients: A Pilot, Retrospective, Real-World Study Volume 5 - Issue 2
Walter Gianni1*, Ilaria Spoletini2, Colangelo Luciano1, Riccardo Nicolucci1, Cristiana Vitale2, Giuseppe MC Rosano2, Gianluca Mairone1 and Giustino Varrassi3
- 1University “La Sapienza”, II Medical Clinic of the Policlinico Umberto I, Rome
- 2IRCCS San Raffaele, Rome
- 3Paolo Procacci Foundation (FPP), Rome
Received: January 28, 2023; Published: February 10, 2023
Corresponding author: Walter Gianni, II Medical Clinic of the Policlinico Umberto I Viale del Policlinico 155, Rome, Italy
DOI: 10.32474/GJAPM.2023.05.000207
Abstract
Introduction: Chronic pain affects up to 70% of the elderly general population. The use of transdermal buprenorphine may help relieve pain, but its use in elderly patients is hindered by concerns of adverse effects. In particular, its possible detrimental effect on the cognitive level throughout mechanisms involving the CNS is unclear. Also, the efficacy of buprenorphine patch on behavioural profile as well as on the functional level has yet to be clarified.
Methods: A 2-month, retrospective, pilot and real-world study was designed to explore the effects of transdermal buprenorphine on chronic pain, cognitive level, behavioural profile and functional abilities in elderly patients with persistent (≥3 months) moderate-severe chronic non-cancer pain (NRS >4). Buprenorphine patch was administered in the new-developed weekly formulation, with 5μg / h release. Cognitive level, behavioural symptoms and functional abilities were assessed at baseline and at the end of the study. Adverse events were recorded. ANOVA within group comparisons were performed.
Results: A total number of 97 patients were enrolled (baseline NRS 6.8±1.8, mean age 81±7) and n=90 patients completed the study. A statistically significant improvement was observed in NRS score and NPI score. No statistically significant changes in MMSE score, ADL/IADL scores were observed. Reported adverse events were all of mild intensity, with a decreasing trend over time.
Conclusion: Despite the short study duration, this pilot study suggests the short-term efficacy of buprenorphine patch in reducing moderatesevere chronic pain and in improving the neuropsychiatric/behavioural profile, with a good safety profile. Patients’ cognitive status was unaffected by the treatment.
Keywords: Behavioural symptoms; Buprenorphine patch; Cognitive level; Elderly; Functional level; Pain; Pilot study
Introduction
Chronic pain is a health problem with a huge impact on the general population, particularly in the elderly [1,2]. It is wellrecognized that the experience of pain has a detrimental impact on the global state of health, involving emotional, relational, social and cultural factors, therefore affecting patient’s quality of life [3]. In particular, chronic non-cancer pain is a condition defined by multiple processes underlying aging. In elderly, it causes impairments at behavioural, functional and cognitive levels [4]. As such, pain is often accompanied by symptoms of anxiety, depression agitation, insomnia and aggression [5] increased medical comorbidities, cognitive disorders, and impairment of autonomy in daily activities [6]. Pain-related impairments can also lead to an increase in care demand, caregiver distress and costs for health system [7]. Although chronic pain is highly represented in the geriatric population, it is often underestimated, underdiagnosed and consequently undertreated, fueling the chronicity of pain itself [6-8]. In fact treatment of chronic pain is hindered by polypharmacy and lack of compliance in this frail population [6]. Among opioids, buprenorphine is considered as the top-line choice for chronic pain treatment in the elderly [9]. However, the use of opioids in clinical practice is limited by fears due to the risk of side effects (opioidophobia) [10,11] Side effects comprise cognitive-behavioural, respiratory, gastrointestinal symptoms, as well as the risk of abuse and dependence. As a consequence, nonsteroidal anti-inflammatory drugs and paracetamol are more often prescribed in these patients, despite a greater risk of adverse effects and numerous contraindications [12].
On the other hand, buprenorphine has optimal pharmacodynamic and pharmacokinetic characteristics, including its agonism-antagonism action on the different subtypes of CNS receptors, representing a therapeutic advantage [13,14]. In particular, transdermal buprenorphine has significantly enhanced the clinical use of the drug, offering continuous drug release [15]. Transdermal formulation is particularly beneficial in elderly patients as it allows a slow increase of plasma concentrations, without a sudden peak, with consequential reduction of adverse events [16]. Clinical trials and large-scale post-marketing studies confirmed the effectiveness of transdermal buprenorphine in treating moderateto- severe non-cancer pain and low incidence of CNS adverse events in patients receiving transdermal buprenorphine [14-16]. However, data in elderly patients are scanty: older adults are generally under investigated in clinical trials and this population is poorly represented in studies investigating pain as well [17]. Beyond clinical trials, data on efficacy/safety of buprenorphine collected from routine clinical care (i.e., “real world data”) should be implemented [18]. Finally, there is preliminary evidence from a former study by our research group with a high-dose [14] that transdermal buprenorphine, starting from a dose of 17.5 μg/h and uptitrated to 35 μg/h (with a 72h-administration route), was associated with a decrease in pain severity without negative effects on the CNS in elderly patients. For all these reasons, we conducted a retrospective, real-life, feasibility, pilot study aimed at investigating efficacy and safety of a novel formulation of buprenorphine patch with a low dosage (starting from a dose of 5 μg/h) and a weekly administration, on chronic pain, cognitive level, behavioural profile and functional abilities in elderly patients with persistent moderate-severe chronic non-cancer pain.
Methods
A total number of 97 patients were consecutively enrolled in the Geriatric Department for chronic pain therapy at the II Medical Clinic of the Policlinico Umberto I in Rome, between October 2019 and March 2020. All patients fulfil the following characteristics: age ≥ 65 years; chronic (≥3 months) non-cancer pain of moderatesevere level (NRS>4). Patients were asked to discontinue previous treatments with analgesic or anti-inflammatory drugs before starting therapy with buprenorphine patch in the weekly formulation, with 5μg / h release. Patients were allowed to increase the dose of buprenorphine by 5 μg /day in absence of clinical benefits after 2 weeks. A 2-month timeframe was considered for the evaluation of the following domains: the efficacy of the drug on pain, the influence on cognitive functions and cognitive profile. Pain and side effects were assessed/recorded at T0 at baseline, T1 at one week (by telephone), T2 at one month, T3 at two months. All the other variables were assessed at T0 (baseline) and at the end of the study (T3, two months).
Instruments
The 0 to 10 NRS is a unidimensional measure of pain intensity in adults [19]. Patients were asked to rate the intensity of their pain using any number between 0 and 10, where 0 is ‘no pain’ and 10 is ‘the strongest or worst pain you can imagine’, on a segmented numeric version of the visual analog scale. The common format is a horizontal bar or line. Katz Index of Independence in Activities of Daily Living (ADL) [20] and Lawton Instrumental Activities of Daily Living (IADL) [21] scales were used to assess functional abilities. The ADL scale is based on the level of independence in performing six daily actions: bathing with a sponge, bath or shower; dressing; toilet use; transferring in and out of a bed or chair; urine and bowel continence; and eating. The IADL scale is based on 7 criteria (use of the telephone, traveling via car or public transportation, food or clothes shopping, meal preparation, housework, medication use, and management of money) and there are two separate scores for males and females. Lower scores in ADL and IADL indicate worse autonomy. Neuropsychiatric Inventory-12 (NPI) [22] was used to assess the following behavioural domains: delusions, hallucinations, agitation/aggression, dysphoria, anxiety, euphoria, apathy, disinhibition, irritability/lability, aberrant motor activity, night-time behavioural disturbances and appetite/eating abnormalities. Mini Mental State Examination (MMSE) [23] was administered to quantitatively assess the cognitive status. It is a widely used screening test of orientation, attention, memory, language and visual-spatial skills.
Statistical Analyses
Within group ANOVAs were used to compare measurements to detect differences during baseline (T0) and end of the study (T3). Values are expressed as mean ± standard deviation. The level of statistical significance was defined as <0.05. Statistical analyses were performed by using the Stat View statistical software package (SAS Institute INC., Cary, NC).
Results
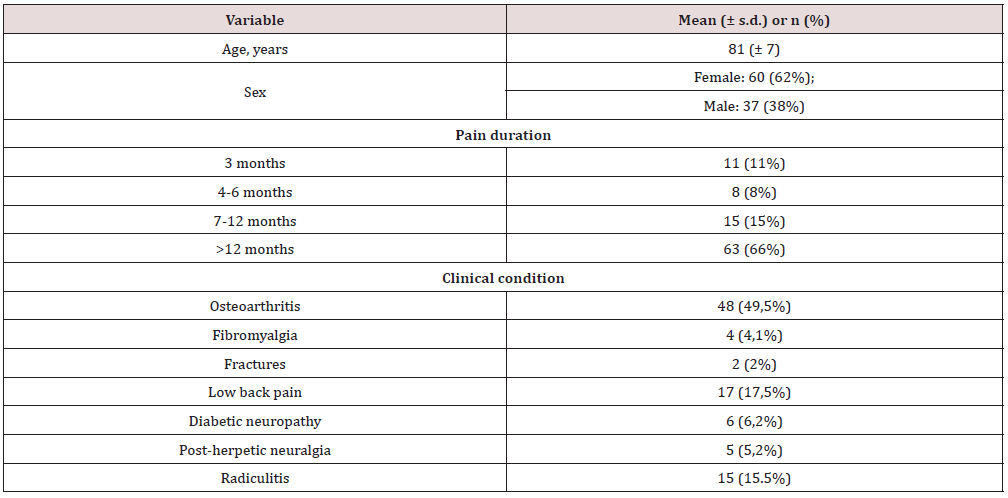
Baseline pain characteristics of the sample (n=97; mean age 81±7) are shown in Table 1 Seven patients dropped out the study due to non-severe side effects (below described). Thus, the final sample consisted of 90 patients (n=56 (62%) women; n=34 (38%) men). Patients who did not complete the study were all “early drops”: n=3 for the development of rash at the site of application of the patch; n=4 due to sweating and lethargy, manifested 3 hours after patch application. Changes over time on pain, behavioural, functional capacities, and cognitive level are shown in at baseline, the mean value of pain intensity was 6.8 ± 1.8 at the NRS. This value significantly decreased at the end of the study period (3 ± 1.5) (p <0.0001). Regarding behavioural profile, a statistically significant improvement was observed from a mean NPI score of 17.0 ± 1 at T0, to 3.8 ± 1.6 at T1 (p <0.0001). As for functional capacities in daily activities, no differences were observed in ADL and IADL scores, (respectively from a mean value of 4 at T0, to 4.5 at T1 (p=ns). Similar results were observed for IADL score, from 4 at T0 to 5 at T1 (p=ns). No statistically significant changes in cognitive status were observed: at T0, the mean value of MMSE score was 23, while, at two months, the mean value was 24 (p=ns). Reported adverse events were all of mild intensity showing a decreasing trend during the study period: dizziness (23% at T1, 8.5% at T3), nausea (17.5% at T1, 2% at T3), drowsiness (15% at T1, 2% at T3), itching at the application site (15% at T1, 6% at T3), dry mouth (15% at T1, 2% at T3), and vomiting (4% at T1 and 0% at T3). Mean dosage of buprenorphine was 12.5 μg /day at T3.
Discussion
This real-life pilot study provides preliminary results on the short-term efficacy of transdermal buprenorphine in reducing moderate-severe chronic pain in elderly patients, consistently with previous reports [14,23,24]. Beyond pain reduction, our study also showed a short-term improvement in the total NPI score, thus providing preliminary evidence that treatment with low-dosage buprenorphine patch may lead to enhanced behavioural profile, without any detrimental effects on cognitive profile. A good safety profile was observed. The reported adverse events were of mild intensity, reversible, showing a progressive reduction over the 2-month observational period. In line with literature [25] one of the most common adverse effects was local skin symptoms, due to the transdermal formulation of the drug. Our data are consistent with results from a recent randomized study [26] investigating the efficacy and safety of transdermal buprenorphine on pain and quality of life of elderly patients with osteoarthritis, which concluded that treatment with buprenorphine patch provided effective analgesia and improvement of quality of life. Longer follow ups are probably needed to appreciate variations on functional capacities. The beneficial effects observed in our study may be explained considered the aforementioned pharmacokinetic and pharmacodynamic proprieties of transdermal buprenorphine, in particular its slow release and constant plasma concentration. Unlike the aforementioned previous report [14] the novel formulation used in this study is a low-dose and weekly type, making it easier to manage in patients who are burdened by polytherapy and consequent lack of compliance [24]. Due to the methodological limitations of this exploratory pilot study, such as its observational design, the absence of a control group and the short study duration, generalizations of the results cannot be drawn. The real word data here provided suggest that buprenorphine patch, in its weekly formulation at 5μg/h, may represent a valuable choice in the treatment of moderate-severe chronic pain in the elderly patient, with a benefit-risk balance in favour of the benefit. However, further studies are needed to substantiate these preliminary results. In particular, randomized clinical trial and larger real-life studies with longer follow ups are warranted.
Data availability
The datasets generated during and/or analyzed during the current study are available. from the corresponding author on reasonable request.
Acknowledgments
The authors are grateful to the Paolo Procacci Foundation for the support in the editorial activities. Rapid service fee is sponsored by Sandoz Italia. This work was supported by Ar. Ger. on onlus.
Compliance with Ethics Guidelines
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Ethics committee approval was not required as this is a retrospective study.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All Authors equally contributed to the final paper.
Funding
This study was supported by the Italian Ministry of Health (Ricerca Corrente) 20/1904.
Conflicts of Interests
None.
References
- Camilloni A, Nati G, Maggiolini P (2021) Chronic non-cancer pain in primary care: An Italian cross-sectional study. Singa Vitae 17(2): 54-62.
- Latina R, De Marinis MG, Giordano F (2019) Epidemiology of chronic pain in the Latium Region, Italy: A cross-sectional study on the clinical characteristics of patients attending pain clinics. Pain Manag Nurs 20(4): 373-381.
- Bernfort L, Gerdle B, Rahmqvist M, Husberg M, Levin L (2015) Severity of chronic pain in an elderly population in Sweden-impact on costs and quality of life Pain 156: 521.
- Frondini C, Lanfranchi G, Minardi M, Cucinotta D (2007) Affective, behavior and cognitive disorders in the elderly with chronic musculoskeletal pain: the impact on an aging population. Arch Gerontol Geriatr 1: 167-171.
- Noroozian M, Raeesi S, Hashemi R (2018) Pain The neglect issue in old people’s life. Maced J Med Sci 6(9): 1773-1778.
- Gianni W, Madaio RA, Di Cioccio L (2010) Prevalence of pain in elderly hospitalized patients. Arch Gerontol Geriatr 51(3): 273-276.
- Zwakhalen SM, Hamers JP, Abu-Saad HH, Berger MP (2006) Pain in elderly people with severe dementia:a systematic review of behavioural pain assessment tools. BMC Geriatr 1: 3.
- Caltagirone C, Spoletini I, Gianni W, Spalletta G (2010) Inadequate pain relief and consequences in oncological elderly patients. Surg Oncol 19: 178-183.
- Pergolizzi J, Böger RH, Budd K (2008) Pain Pract 8(4): 287-313.
- Gianni W, Ceci M, Bustacchini S (2009) Opioids for the treatment of chronic non-cancer pain in older people. Drugs Aging 1: 63-73.
- Vadivelu N, Hines RL (2008) Management of chronic pain in the elderly: focus on transdermal buprenorphine. Clin Interv Aging 3(3): 421-430.
- American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 57(8): 1331-1346.
- Paladini A, Fusco M, Coaccioli (2009) Chronic Pain in the Elderly the Case for New Therapeutic Strategies. Pain Physician 18: 863-876.
- Gianni W, Madaio AR, Ceci M (2011) Transdermal buprenorphine for the treatment of chronic noncancer pain in the oldest old. J Pain Symptom Manage 41(4): 707-714.
- Kress HG (2009) Clinical update on the pharmacology, efficacy and safety of transdermal buprenorphine. Eur J Pain 13(3): 219-230.
- Gudin J, Fudin J (2020) A narrative pharmacological review of Buprenorphine: A unique opioid for the treatment of chronic pain. Pain Ther 9(1): 41-54.
- Carvalho do Nascimento PR, Ferreira ML, Poitras S, Bilodeau MJ (2019) Exclusion of Older Adults from Ongoing Clinical Trials on Low Back Pain: A Review of the WHO Trial Registry Database. J Am Geriatr Soc 67(3): 603-608.
- Blonde L, Khunti K, Harris SB (2018) Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther (11): 1763–1774.
- Farrar JT, Young Jr JP, LaMoreaux L (2001) Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94(2): 149-158.
- Katz S, Downs TD, Cash HR, Grotz RC (1970) Progress in development of the index of ADL. Gerontologist 10(1): 20-30.
- Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9(3): 179-186.
- Cummings JL (1997) The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 48: 10-16.
- Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3): 189-198.
- Widenka M, Leppert W (2020) Assessment of analgesic effects of different initial doses of transdermal buprenorphine in the treatment of chronic pain in the elderly diagnosed with osteoarthritis. J Physiol Pharmacol 71(5).

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...