Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1403
Case Report(ISSN: 2644-1403) 
Awake Brain Surgery in Children: Case Report and Review of Literature Volume 4 - Issue 2
Atroune L1, Bouaita K1, Benhafri A1, Habchi N1, Bennaleg S1 and Djaffer M1
- 1Department of neurosurgery CHU Mustapha Bacha Algiers, Algeria
Received:March 20, 2021; Published: April 5, 2021
Corresponding author: Atroune L, Department of neurosurgery CHU Mustapha Bacha Algiers, Algeria
DOI: 10.32474/GJAPM.2021.04.000184
Abstract
Awake craniotomy is today a common and effective practice worldwide and in Algeria, however it remains a rare surgical procedure in children due to age and psychological aspects that hinder with its feasibility and limits its application. Despite extensive literature in the adult population awake brain surgery remains poorly reported in the pediatric population. The aim of this article is to report a case of a 15 years old child who was treated in our department with excellent outcome.
Keywords: Awake brain surgery; Child, Pediatric; Awake craniotomy
Introduction
The purpose of awake brain surgery (ABS) with cortical stimulation is to help identify and preserve eloquent areas during cortical and subcortical’ s tumor resections, during surgery for arteriovenous malformations, and for respective epilepsy surgery [1]. The overall outcome in adult population has been extensively studied and reported in several patients’ cohorts [2,3]. While ABS became a standard of care in adults, ABS in pediatric patients finds obstacles in an assumed increased psychological fragility in children and age-related cooperation capacity interfering with feasibility and psychological outcome [4,5].
Case Report
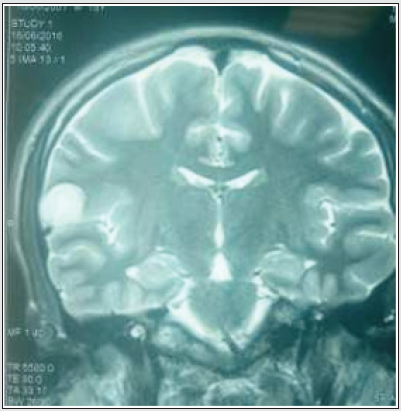
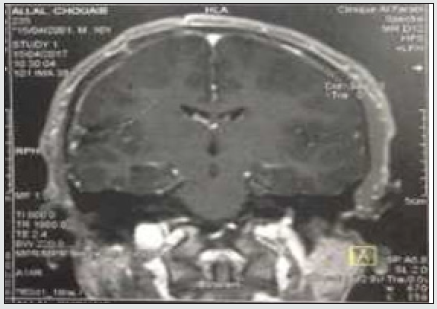
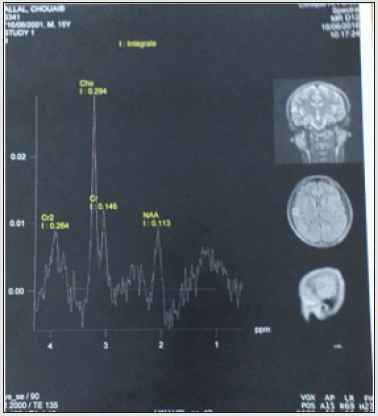
A 15-year-old male left-handed patient with a long-standing history of drug-resistant epilepsy was referred to our unit for surgical resection of what was presumed to be a low-grade glioma in the right Rolandic operculum. For the previous 8 months, he had been experiencing 6 -7 seizures per month despite appropriate dosages of valproic acid and carbamazepine. Physical examination did not reveal any neurological disorder; however, he did have a moderate headache. Neuropsychological tests showed good administration of the cognitive functions essential to the control and achievement of motivated behaviors, as well as good planning, judgment and decision-making capacities, self-monitoring, and mental flexibility. There was no deficit of linguistic activities (no aphasia). The episodic system and short-term memory were preserved. MRI imaging revealed an 18 x18.7x18 mm intra-axial cortico-subcortical right parietal nodular formation, oval, with clear limits, T2 hyperintense, T1 iso-intense, with intra-lesional annular enhancement. Spectroscopy showed a choline peak with an increase in the choline/creatine ratio (2.02) and the choline / Naa ratio (2.60) which points to a low-grade glial tumor (Figures 1,2,3).
A preoperative multi-session conditioning with the neuropsychologist had been followed for 3 months. The patient was dressed in theatre attire and brought into the theatre on a theatre trolley. He was then transferred onto the theatre bed and positioned in the same manner as he would be for the actual surgery. His head was placed on a horseshoe headrest, but not pinned. A blood pressure cuff, pulse oximeter, nasal cannula with oxygen flow, and calf pumps were applied. He was then draped precisely as he would have been for the procedure. Theatre lighting was set as it would be for the surgical case. The speech therapist spoke with him through the procedure, enquiring repeatedly on what would make him feel more comfortable and less stressed. The surgical team explained to him the steps that he would be put through on the day of surgery.
Anesthetic Technique
An asleep-awake-asleep technique was planned and employed.
Anesthesia was induced and maintained with total intravenous
anesthesia (TIVA) using a propofol target control anesthesia model
(Paedfusor). A laryngeal mask airway (LMA) was placed, and the
patient breathed spontaneously with pressure support provided
from the ventilator. The choice of an LMA over an endo-tracheal
tube was in order to avoid any injury occurring to the patient
due to coughing on extubation while he was in head pins, and an
LMA was easy to put back at the end of the awake phase. Standard
monitoring was used. With close communication from the surgeon,
the propofol infusions were discontinued for the awake part of
the craniotomy. At the time of discontinuation of the infusions, in
preparation for the wake up, propofol dose was at an estimated
plasma site concentration of 4.2mcg/ml (dose titrated to clinical
effect, entropy, and the neurophysiological monitoring). The
patient opened his eyes, and the LMA was removed 14 minutes
after stopping propofol.
After an initial period of some disorientation, he was able to
respond properly to questions. At the end of the awake period,
which lasted a total of 83 minutes, the propofol infusions have
been started again, the patient was anesthetized, and the LMA was
reinserted to maintain airway patency.
Surgical Technique
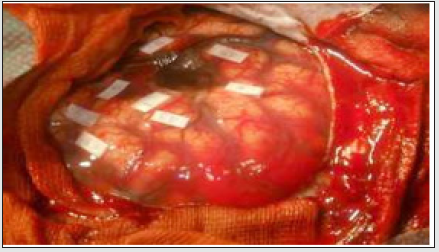
An asleep-awake-asleep technique was employed. The patient was positioned in a left lateral position. The head was placed in a left lateral position on a Mayfield head holder. A right frontotemporal arciform incision of the scalp was done. the bone flap was turned, and the dura was infiltrated with local anesthetic (2% xylocaine). At this point, the patient was woken up and the LMA was removed. When the child was able to communicate with the linguist and was calm and cooperative, the dura was opened. Intraoperative ultrasound neuronavigational was used to identify tumor boundaries. It was located under the Sylvian fissure. A bipolar electrode, delivering a biphasic current, was applied, and positive and negative motor and speech mapping was performed (Figure 4). The tumor was in the functional areas of language and facial motricity. For sites involved in language function, spontaneous speech was assessed by the speech therapist. After gross total tumor resection, the child was anesthetized, and the LMA was reinserted. The post-operative evolution was favorable both clinically and radiologically. The child was seen four weeks postoperatively. A follow-up MRI scans revealed gross total removal. Histology confirmed allow grade glioma. The patient had no further seizures, and no postoperative deficits. He reported having no pain or anxiety during the procedure. He had no significant negative emotions related to the surgery.
Discussion
The principle of awake brain surgery (ABS) evolved since the
first maps of the sensory and motor cortices of the brain have been
developed by Wilder Penfield [6]. It became a surgical approach
that helps to identify and preserve eloquent areas during cortical
and subcortical tumor resections, during surgery for arteriovenous
malformations, and for respective epilepsy surgery. Awake
surgery with direct cortical stimulation is the gold standard for
identifying eloquent cortical sites in the adult population [7,8].
Only a few small series, however, have been published regarding
this treatment modality in children [9,10]. The purpose of ABS with
electro cortical mapping is to minimize neurological morbidity
while performing the best possible cyto-reduction within eloquent
areas [11-13]. The use of awake protocols in pediatric brain surgery
remains limited due to assumed increased psychological fragility
and vulnerability. Moreover, the pediatric population presents
specific challenges related to cooperation, full understanding,
and managing concomitant anxiety [14]. With decreasing age, the
child’s ability to cooperate, understand, and manage the stressful
surgical environment of an awake craniotomy becomes more and
more relevant [15,16]. Some authors [17] consider 11 to12 years
of age being the absolute minimum, whereas other authors have
successfully applied this technique in children as young as 8years
of age [18,19].
In 1954, Pasquet noted that “uncooperative adults and
children under 10 years” will not tolerate the application of local
anesthesia, scalp incision, and craniotomy [20]. Contrarily, Klimek
et al. demonstrated in their case report that an awake craniotomy is
feasible and can be performed safely even in very young patients, and
it seems unacceptable to maintain an age restriction [21]. Several
studies confirmed that the extent and the quality of preoperative
psychological preparation and intra-operative support have a
relevant impact on the psychological experience. Furthermore,
they correlate with neuropsychological outcome [22,6,23]. Thus,
attempts should be made to establish eligibility criteria for the
pediatric age group and to increase the overall psychological
support by qualified psychologists. According to Girvin [24], “the
psychological preparedness of the patient is the most important
consideration” for a successful awake craniotomy. Despite this
degree of preparation, at least 10–15% of adult patients still
report severe anxiety during the procedure [25]. The preparation
of a child for awake surgery is more difficult, firstly to ensure that
the child will be cooperative and safe during the awake part of the
surgery and also to minimize the anxiety and posttraumatic stresslike
symptoms following an awake craniotomy [26]. Riquin et al.
[18] describe a very simple preparation phase including having the
child examined by a child psychiatrist, hypnotic conditioning of the
child, offering the child an opportunity to meet another child who
has been operated on while awake, showing the patient pictures
and a video describing the atmosphere of the operating room, a
visit to the operating room, and a chance to meet the surgical and
anesthetic team. Using mental diversion techniques (virtual reality
glasses) can be very helpful before and during the surgery.
Laura-Nanna Lohkamp an Al. [2] have established some
preoperative recommendations for a successful ABS in child,
but precise protocols on psychological/neuropsychological
eligibility criteria that represent an important tool in the complex
multidisciplinary preparation of children for ABS remain unsettled.
The accuracy of intraoperative ultrasound (IoUS) was initially
tested in laboratory setting and lately confirmed in surgical
theatres. Lindseth et al. described an accuracy of 1.40±0.45 mm
(arithmetic mean) for ultrasound-based neuron avigation system,
and highlighted that improper probe calibration was the major
contributor to these accuracy errors. Of note, by simultaneously
recording Doppler and B-mode images, this accuracy can be further
improved; Morin et al. quantified this increase in over 10%, with a
67% correction of brain-shift. Similarly, by adopting Doppler IoUS
images acquired on the surface of the dura to correct brain-shift
and other sources of registration in accuracies, Chen et al. found
that target registration errors (TRE) were less than 2.5 mm in
more than 90%of cases. These figures were confirmed in clinical
scenarios were the TREs was on average inferior to the 3 mm of
standard neuronavigational based on preoperative MRI scans alone
[27].
Conclusion
Awake brain surgery is beneficial to an efficient tumor resection with simultaneous preservation of neurological functions. It requires, however, a serious psychological preparation of the child. Neuropsychological testing before and after surgery is essential to determine cognitive outcome, which can be altered in a minority of patients.
References
- Jason Labuschagne, Clover-Ann Lee, Denis Mutyaba, Tatenda Mbanje,and Cynthia Sibanda (2020) ‘Awake Craniotomy in a Child: Assessment of Eligibility with a Simulated Theatre Experience’, Volume 2020.
- Laura-Nanna Lohkamp, Carmine Mottolese, Alexandru Szathmari, Ludivine Huguet,Pierre-Aurelien Beuriat, Irène Christofori, Michel Desmurget, Federico Di Rocco (2019) ‘Awake brain surgery in children-review of the literature and state-of-the-art’ Child's Nervous System 35: 2071-2077.
- Serletis D, Bernstein M (2007) Prospective study of awake craniotomy used routinely and nonselectively for supratentorial tumors. J Neurosurg 107: 1-6.
- Gupta DK, Chandra PS, Ojha BK, Sharma BS, Mahapatra AK, et al. (2007) Awake craniotomy versus surgery under general anesthesia for resection of intrinsic lesions of eloquent cortex-a prospective randomised study. Clin Neurol Neurosurg 109: 335- 343.
- Trevisi G, Roujeau T, Duffau H (2016) Awake surgery for hemispheric low-grade gliomas: oncological, functional and methodological differences between pediatric and adult populations. Childs Nerv Syst 32: 1861-1874.
- Delion M, Terminassian A, Lehousse T, Aubin G, Malka J, et al. (2015) Specificities of awake craniotomy and brain mapping in children for resection of supratentorial tumors in the language area. World Neurosurg 84: 1645-1652.
- Snyder PJ, Whitaker HA (2013) Neurologic heuristics and artistic whimsy: the cerebral cartography of Wilder Penfield. J Hist Neurosci 22: 277-291.
- Ojemann G, Ojemann J, Lettich E, Berger M (1989) “Cortical language localization in left, dominant hemisphere.” Journal of Neurosurgery 71(3): 316-326.
- Potters JW, Klimek M (2015) “Awake craniotomy,” Current Opinion in Anaesthesiology 28(5): 511-516.
- Akay A, Ruks¸en M, Çetin HY, Seval HO, Islekel S (2016) “Pediatric awake craniotomy for brain lesions,” Pediatric Neurosurgery 51(2): 103-108.
- Tobias J, Jimenez D (1997) “Anaesthetic management during awake craniotomy in a 12-year-old boy,” Pediatric Anesthesia 7(4): 341-344.
- Hervey-Jumper SL, Li J, Lau D,Molinaro AM, Perry DW, Meng L,et al. (2015) Awake craniotomy to maximize glioma resection: methods and technical nuances over a 27-year period. J Neurosurg 123: 325-339.
- Szelenyi A, Bello L, Duffau H, Fava E, Feigl GC, et al. (2010) Intraoperative electrical stimulation in awake craniotomy: methodological aspects of current practice. Neurosurg Focus 28: E7.
- Freyschlag CF, Duffau H (2014) Awake brain mapping of cortex and subcortical pathways in brain tumor surgery. J Neurosurg Sci 58: 199-213.
- Everett LL, van Rooyen IF, Warner MH, Shurtleff HA, Saneto RP, et al. (2006) Use of dexmedetomidine in awake craniotomy in adolescents: report of two cases. Paediatr Anaesth 16: 338-342.
- Berger MS (1996) “The impact of technical adjuncts in the surgical management of cerebral hemispheric low-grade gliomas of childhood,” Journal of Neuro-Oncology 28(2-3): 129-155.
- Riquin E, Dinomais M, Malka J (2017) “Psychiatric and psychologic impact of surgery while awake in children for resection of brain tumors,” World Neurosurgery 102: 400-405.
- Balogun JA, Khan OH, Taylor M (2014) “Pediatric awake craniotomy and intra-operative stimulation mapping,” Journal of Clinical Neuroscience 21(11): 1891-1894.
- Delion M, Terminassian A, Lehousse T (2015) “Specificities of awake craniotomy and brain mapping in children for resection of supratentorial tumors in the language area,” World Neurosurgery 84(6): 1645- 1652.
- Pasquet A (1954) Combined regional and general anesthesia for craniotomy and cortical exploration. II. Anesthetic considerations. Curr Res Anesth Analg 33: 156-164.
- Klimek M, Verbrugge SJ, Roubos S, van der Most E, Vincent AJ, et al. (2004) Awake craniotomy for glioblastoma in a 9-year-old child. Anaesthesia 59: 607-609.
- Riquin E, Dinomais M, Malka J, Lehousse T, Duverger P, et al. (2017) Psychiatric and psychological impact of surgery while awake in children for resection of brain tumors. World Neurosurg 102: 400- 405.
- Santini B, Talacchi A, Casagrande F, Casartelli M, Savazzi S, et al. (2012) Eligibility criteria and psychological profiles in patient candidates for awake craniotomy: a pilot study. J Neurosurg Anesthesiol 24: 209-216.
- Girvin JP (1986) “Neurosurgical considerations and general methods for craniotomy under local anesthesia,” International Anesthesiology Clinics 24(3): 89-114.
- Milian M, Tatagiba M, Feigl GC (2014) “Patient response to awake craniotomy - a summary overview,” Acta Neurochirurgica 156(6): 1063-1070.
- Milian M, Luerding R, Ploppa A (2013) ““Imagine your neighbor mows the lawn”: a pilot study of psychological sequelae due to awake craniotomy,” Journal of Neurosurgery 118(6): 1288-1295.
- Mario Ganau, Gianfranco K Ligarotti, Vasileios Apostolopoulos (2019) ‘Real-time intraoperative ultrasound in brain surgery: neuronavigation and use of contrast-enhanced image fusion’ Quant Imaging Med Surg 9(3): 350-358.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...