Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1403
Case Report(ISSN: 2644-1403) 
Apical Takotasobo Cardiomyopathy Post-Covid Pneumonia Associated with Changeable Bifascicular Block Volume 4 - Issue 2
Yasser Mohammed Hassanain Elsayed?1*
- 1Critical Care Unit, Al-Rodah Central Hospital, Damietta Health Affairs, Egyptian Ministry of Health (MOH), Damietta, Egypt
Received:April 08, 2021; Published: May 04, 2021
Corresponding author: Yasser Mohammed Hassanain Elsayed, Critical Care Unit, Al-Rodah Central Hospital, Damietta Health Affairs, Egyptian Ministry of Health (MOH), Damietta, Egypt
DOI: 10.32474/GJAPM.2021.04.000186
Abstract
Rationale: A novel COVID-19 with severe acute respiratory syndrome had emerged in Wuhan, China in December 2019. The presence of takotasobo cardiomyopathy in COVID-19 patients is an extremely rare and commonly recognized post severe stress. The changeable bifascicular block is also very rare to be present after COVID-19 with the severe acute respiratory syndrome. Interestingly, the combination of COVID-19 pneumonia with an apical takotasobo cardiomyopathy and changeable bifascicular block has a worthy risk impact on both morbidity and mortality of COVID-19 patients. Patient concerns: An elderly carpenter male COVID-19 pneumonic patient was admitted to the critical care unit with dyspnea and tachypnea post-severe emotional stress with evidence of acute myocardial infarction, cardiomyopathy, and changeable bifascicular block. Diagnosis: Apical takotasobo cardiomyopathy post- COVID pneumonia associated with changeable bifascicular block. Interventions: Chest CT scan, electrocardiography, oxygenation, and echocardiography. Outcomes: Gradual clinical, and radiological improvement had happened. Lessons: An apical ballooning after emotional stress in recent COVID pneumonia is an indicator for apical takotasobo cardiomyopathy. The S-T segment elevations with non-specific T-wave inversions and changeable bifascicular block in apical ballooning may be a hallmark for the existence of apical takotasobo cardiomyopathy. The changeable non-specific T-wave inversions and bifascicular block is a recommended interesting issue for further wide-study.
Keywords: COVID-19; Coronavirus; Pneumonia; Takotasobo cardiomyopathy; Changeable bifascicular block; Heart block
Abbreviations:ACS: Acute Coronary Syndrome, CBC: Complete Blood Count, COVID-19: Coronavirus Disease 2019, ECG: Electrocardiography, IHD: Ischemic Heart Disease, IV: Intravenous, IVB: Intravenous Bolus, LAFB: Left Anterior Fascicular Block, LBBB: Left Bundle Branch Block, LPFB: Left Posterior Fascicular Block, LV: Left Ventricular, O2: Oxygen, RBBB: Right Bundle Branch Block, SGOT: Serum Glutamic-Oxaloacetic Transaminase, SGPT: Serum Glutamic-Pyruvic Transaminase, TTC: Takotsubo Cardiomyopathy, VR: Ventricular Rate
Introduction
The primary presentation of a novel Coronavirus-2 (COVID-19) that is resulting in severe acute respiratory syndrome (SARS) had emerged in Wuhan, China in December 2019 [1]. Takotsubo cardiomyopathy (TTC) is non-ischemic cardiomyopathy in which there is an unexpected transient weakening of the cardiac muscle [2]. It commonly emerges post a severe physical or emotional stressor. If it is caused by the latter, then the condition is named broken heart syndrome [3]. Physical stressors (41-50%) that can cause TTC are serious illness, sudden hypotension, surgery, or medical procedure such as cardiac stress test, severe pain, asthma attack, sepsis, shock, and pheochromocytoma. The emotional stressors (26-30%) such as bereavement, domestic violence, receiving, car or other accident, bad news, sudden loss, illness, or injury of a close relative, friend, violent argument, economic loss, severe fear, public speaking, a sudden surprise, divorce, or the loss of a job [4-7]. The pathophysiology is not still unknown, but an unexpected huge surge of catecholamines after ultimate stress or a tumor secreting these chemicals is suggestive theory [8]. Takotsubo cardiomyopathy is imitative for the acute coronary syndrome (ACS). It is associated with reversible left ventricular (LV) apical ballooning in the absence of angiographic-marked coronary artery stenosis. In Japanese, “tako-tsubo” means “fishing pot for trapping octopus,” because the LV of a patient diagnosed with this condition resembles that shape [9]. Mayo Clinic Researchers (2004) [10] proposed some diagnostic criteria: A. temporal hypokinesis, akinesis, or dyskinesis in the left ventricular mid segments with or without apical involvement; regional wall motion abnormalities (RWMA) that extend beyond a single epicardial vascular distribution; and frequently, but not always, a stressor; (B) the absence of obstructive ischemic heart disease or angiographic evidence of acute plaque rupture; B. new ECG abnormalities (ST-segment elevation and/ or T-wave inversion) or modest elevation in cardiac troponin, and C. the absence of pheochromocytoma and myocarditis. Patients commonly present with chest pain, have ST-segment elevation myocardial infarction (STEMI) on electrocardiography (ECG) with a consistent elevation of cardiac enzymes [11]. The prognosis of patients with TTC is generally favorable and excellent unless there are fatal complications such as left ventricular free wall rupture10. Supportive treatment leads to spontaneous rapid recovery in nearly all patients10. The prognosis is, and a recurrence occurs in <10% of patients10. There is no standard treatment for TTC. Physicians mostly recommend standard medications in heart failure e.g., aspirin, beta-blockers, ACE inhibitors, and diuretics [5]. Bifascicular block clinically takes one of two electrocardiographic forms: Right bundle branch block (RBBB) with a left anterior fascicular block (LAFB) with left axis deviation (LAD). RBBB and left posterior fascicular block (LPFB), with right axis deviation (RAD). Bifascicular block affects conduction retardation below the atrioventricular node (AVN) in two of the three fascicles. Ventricular conduction is still via the single remaining fascicle. The ECG will be showing RBBB plus either left or right axis deviation. RBBB with LAFB is the most frequent pattern. This is due to a single coronary artery blood supply (LAD) to the anterior fascicle. RBBB with LPFB is less common due to a dual blood supply (right and left circumflex arteries), and this combination may be associated with more extensive underlying cardiac pathology [12]. The common causes of bifascicular heart block are IHD, anterior MI, hypertension, congenital heart disease, Lenègre-Lev disease, aortic stenosis, and hyperkalemia [12]. Elsayed et al. (2019) [13] reported a case of acute myocardial infarction in the presence of with changeable trifascicular heart block. Some authors describe the left bundle branch block (LBBB) as a bifascicular block, as it may indicate LAFB with LPFB. However, clinically the term bifascicular block is reserved for RBBB with either LAFB or LPFB [12].
Case Presentation
A 69-year-old married Carpenter Egyptian male patient was admitted to the critical care unit (ICU) with acute tachypnea and dyspnea. Fatigue and dizziness were associated symptoms. He gave a recent history of confirmed COVID-19 pneumonia 7 days ago. He was managed at the central hospital. Currently, he had a history of severe emotional stress. There was an old history of diabetes and hypertension. Upon general physical examination, generally, the patient was tachypneic, distressed, with an irregular pulse rate (sinus arrhythmia of VR of 72), blood pressure (BP) of 110/70 mmHg, respiratory rate of 34 bpm, the temperature of 36.7 °C, and pulse oximeter of oxygen (O2) saturation of 91%. There were fine bilateral basal crepitations and bilateral pitting lower limb edema. No more relevant clinical data were noted during the clinical examination. The patient was initially treated in ICU as acute myocardial infarction, heart failure, and COVID-19 pneumonia. The patient was treated with O2 inhalation by O2 cylinder (100%, by nasal cannula, 5L/min), aspirin 4 tablets (75 mg then once daily), clopidogrel 4 tablets (75 mg then once daily), and streptokinase IVI (1.5 million units over 60 min). Low dose captopril tablet (12.5 mg once daily) and low dose furosemide tablet (20 mg once daily), cefotaxime; (1000 mg IV every 8hours), azithromycin (500 mg PO single daily dose) was added. The patient was daily monitored for temperature, pulse, blood pressure, and O2 saturation. The initial ECG on presentation showing LBBB with LPFB with sinus arrhythmia of VR of 73, and ST-segment elevation and pathological Q-wave in anterior leads (Figure 1A). The initial complete blood count (CBC); Hb was 13.3 g/dl, RBCs; 4.60*103/ mm3, WBCs; 13.3*103/mm3 (Neutrophils; 79.8 %, Lymphocytes: `10.3%, Monocytes; 9.9%, Eosinophils; 0% and Basophils 0%), Platelets; 218*103/ mm3. S. Ferritin was high; 434 ng/ml. D-dimer was high (578 ng/ml). CRP was high; 13 g/dl. LDH was high; 345 U/L. Total bilirubin was normal (0.6 mg/dl). S. albumen was normal (4.4 gm/dl) SGPT was high; 98 U/L, SGOT was normal; 361 U/L. Serum creatinine showed mild elevation; 1.3 mg/dl and blood urea; showed mild elevation; 73 mg/dl was high. RBS was 134 mg/ dl. Ionized calcium was mildly low; 0.99 mmol/L. The troponin test was positive (7.1 ng/ml).
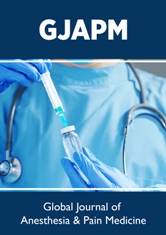
After 9 days of management; RBS was normal; 102 mg/dl. CBC; Hb was 12.7 g/dl, RBCs; 4.29*103/mm3, WBCs; 9.84*103/ mm3 (Neutrophils; 66.3 %, Lymphocytes:29.7%, Monocytes; 3%, Eosinophils; 1% and Basophils 0%), Platelets; 194*103/ mm3. Serum ferritin was normal; 216 ng/ml. D-dimer was normal (284 ng/ml). CRP was negative (5.1 g/dl). LDH was still high; 506.94 U/L. SGPT was normal; 34 U/L, SGOT was normal; 42 U/L. Serum creatinine; 1.2 mg/dl and blood urea; 37.1 mg/dl were normal. Ionized calcium was normal; 1.1 mmol/L. The troponin test had become negative. The first chest CT scan was done on presentation showing bilateral multiple patchy ground-glass pulmonary consolidations (Figure 2A), with apical ballooning on the coronal section (Figure 2B), and cardiac“tako-tsubo” picture on the plain CXR film, and bilateral ground-glass pulmonary consolidations (Figure 2C). Serial ECG tracings were done. ECG tracing was taken within 5 days of the ICU admission showing RBBB with LAFB and sinus arrhythmia of VR of 76, and ST-segment elevation and pathological Q-wave in anterior leads (V1-6). There is T-waves inversion in anterior leads (I, aVL, V1,3 and, V4 (Figure 1B). ECG tracing was taken within 7 days of the ICU admission showing RBBB with LPFB and sinus arrhythmia of VR of 80 with ST-segment elevation and pathological Q-wave in anterior leads (V1-6). There is T-waves inversion in anterior leads (II, III, aVF, and V1, 3, 4, 5, and V6 (Figure 1C). ECG tracing was taken within 9 days of the ICU admission showing RBBB with LPFB and sinus arrhythmia of VR of 85 and ST-segment elevation and pathological Q-wave in anterior leads (V1-6). There is T-waves inversion in anterior leads (II, III and, aVF (Figure 1D). The chest CT scan was done within 5 days of the ICU admission showing bilateral multiple patchy ground-glass pulmonary consolidations (Figure 3A), and cardiac“tako-tsubo” picture on the plain CXR film, and bilateral ground-glass pulmonary consolidations (Figure 3B). Echocardiography was done within 11 days of the ICU admission showed akinetic apical ballooning with systolic dysfunction and EF of 25% (Figure 3C). Apical takotasobo cardiomyopathy post-COVID pneumonia associated with changeable bifascicular block was the most probable diagnosis. Within 9 days of the above management, the patient finally showed nearly complete clinical, radiological, and laboratory improvement. The patient was continued on aspirin tablet (75 mg, once daily), low dose captopril tablet (12.5 mg once daily) and, low dose furosemide tablet (20 mg once daily) with further recommended cardiac and chest follow up.
Figure 1: Serial ECG tracings; A. tracing was done on the initial ECG on presentation showing LBBB with LPFB with sinus arrhythmia of VR of 73 (lime blue and, green arrows ), and ST-segment elevation and pathological Q-wave in anterior leads (V1-6; red and brown arrows). There are tremor artifacts (black arrows). B. tracing was taken within 5 days of the ICU admission showing RBBB with LAFB with sinus arrhythmia of VR of 76 (lime blue and, purple arrows ), and ST-segment elevation and pathological Q-wave in anterior leads (V1-6; red and brown arrows). There is T-waves inversion in anterior leads (I, aVL, V1,3 and, V4; golden arrows). C. tracing was taken within 7 days of the ICU admission showing RBBB with LPFB with sinus arrhythmia of VR of 80 (lime blue and, purple arrows ) with ST-segment elevation and pathological Q-wave in anterior leads (V1-6; red and brown arrows). There is T-waves inversion in anterior leads (II, III, aVF, and V1, 3, 4, 5, and V6; golden arrows). D. tracing was taken within 9 days of the ICU admission showing RBBB with LPFB with sinus arrhythmia of VR of 85 (lime blue and, purple arrows and ST-segment elevation and pathological Q-wave in anterior leads (V1-6; red and brown arrows). There is T-waves inversion in anterior leads (II, III and, aVF; golden arrows).

Figure 2: Chest CT scan was done on the initial presentation showing bilateral multiple patchy ground-glass pulmonary consolidations (A; lime arrows) with apical ballooning on the coronal section (B; golden arrows), and cardiac“tako-tsubo” picture on the plain CXR film (C; golden arrows) and bilateral ground-glass pulmonary consolidations (lime arrows).
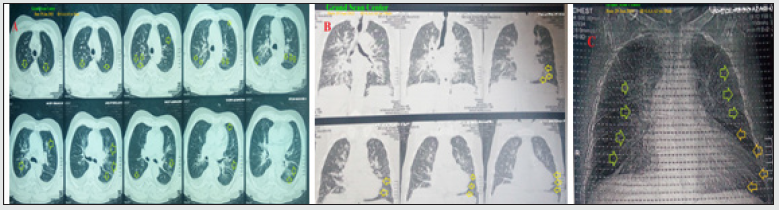
Figure 3: A. chest CT scan was done within 5 days of the ICU admission showing bilateral multiple patchy ground-glass pulmonary consolidations (lime arrows). B. Plain CXR film was done within 5 days of the ICU admission cardiac“takotsubo” picture (golden arrows). C. Echocardiography was done within 11 days of the ICU admission showing akinetic apical ballooning (lime arrows) with an EF of 25%.
Discussion
Overview
a. An elderly carpenter male COVID-19 pneumonic patient was admitted to the critical care unit with dyspnea and tachypnea post-severe emotional stress with evidence of acute myocardial infarction, cardiomyopathy, and changeable bifascicular block.
b. The objective primary for my case study was the presence of COVID-19 pneumonia, ST-segment elevations, non-specific T-wave inversions, changeable bifascicular block, and apical takotasobo cardiomyopathy.
c. The secondary objective for my case study was the question of; How did you manage the case?
d. There was a history of confirmed the COVID-19 case.
e. The presence of direct contact to confirm the COVID-19 case, and bilateral ground-glass consolidation on top of acute tachypnea will strengthen the COVID-19 diagnosis.
f. The dyspnea, tachypnea, fine bilateral basal crepitations, and bilateral pitting lower limb edema are highly suggestive of systolic dysfunction.
g. An associated ST- segment elevations, non-specific T-wave inversions, changeable bifascicular block, and apical ballooning had happened after emotional stress in recent COVID pneumonia.
h. The emotional stress rather than the psychological impact of recent COVID pneumonia may be trigger factors for this apical cardiomyopathy.
i. The changeable non-specific T-wave inversions and bifascicular block is unknown mechanism.
j. Acute ST-segment elevations myocardial infarction is the main differential diagnosis in the current case study. Despite there were positive troponin tests and ST-segment elevations but the other criteria against the diagnosis of infarction.
k. The cause of transient elevation of SGPT, SGOT, serum creatinine, and blood urea is unknown. COVID-19 infection itself and drug-inducing were suggested mechanisms.
l. I can’t compare the current case with similar conditions. There are no similar or known cases with the same management for near comparison.
m. The only limitation of the current study was the unavailability of the catheter lab for diagnosis confirmation.
Conclusion and Recommendation
a. An apical ballooning after emotional stress in recent COVID pneumonia is an indicator for apical takotasobo cardiomyopathy.
b. The S-T segment elevations with non-specific T-wave inversions and changeable bifascicular block in apical ballooning may be a hallmark for the existence of apical takotasobo cardiomyopathy.
c. The changeable non-specific T-wave inversions and bifascicular block is a recommended interesting issue for further widestudy.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgment
I wish to thank my wife to save time and improving the conditions for me and the critical care unit nurses who make extra- ECG copies for helping me.
References
- Zhu N, Zhang D, Wang W, Xingwang Li, Bo Yang, et al. (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727-733.
- Zamir M (2005) The Physics of Coronary Blood Flow. Springer Science and Business Media pp. 387.
- Mayo Clinic team (2006) Mayo Clinic Research Reveals Broken Heart Syndrome Recurs in 1 of 10 Patients. Medical News Today, Medi Lexicon International Ltd.
- Jelena-Rima G, Ilan SW, Abhiram P, Scott S, Keigo D, et al. (2018) International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. European Heart Journal 2(39) (22): 2032-2046.
- Harvard Women's Health Watch (2020) Takotsubo cardiomyopathy (broken-heart syndrome).
- Jimenez S, Francisco E (2013) Initial clinical presentation of Takotsubo cardiomyopathy with-a focus on electrocardiographic changes: A literature review of cases. World Journal of Cardiology 5(7): 228–241.
- Ingo E, Florian VKB, Peter B, Locopo C, Kai M, et al. (2011) Clinical Characteristics and Cardiovascular Magnetic Resonance Findings in Stress (Takotsubo) Cardiomyopathy. JAMA. 2011;306 (3): 277–286. DOI:10.1001/jama.2011.992. ISSN 0098-7484.
- Tavazzi G, Zanierato M, Via G, Iotti GA, Procaccio F (2017) Are Neurogenic Stress Cardiomyopathy and Takotsubo Different Syndromes with Common Pathways? Etiopathological Insights on Dysfunctional Hearts. JACC Heart Failure 5(12): 940-942.
- Virani SS, Khan AN, Mendoza CE, Ferreira AC, de Marchena E (2007) Takotsubo cardiomyopathy, or broken-heart syndrome. Tex Heart Inst J 34(1): 76-79.
- Prasad A, Lerman A, Rihal CS (2008) Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J 155(3): 408-417.
- Tomich EB (2019) Takotsubo (Stress) Cardiomyopathy (Broken Heart Syndrome).
- Ed Burns (2021) Bifascicular Block.
- Elsayed YMH (2019) Acute myocardial infarction associated with right bundle branch block and changeable trifascicular block: a case report. J Acute Dis 8(5): 215-220.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...