Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2644-1403
Research Article(ISSN: 2644-1403) 
Anesthesia For Endoscopic Spine Surgery Of The Spine In An Ambulatory Surgery Center Volume 3 - Issue 5
João Abrão1, Álvaro Dowling2, Jorge Felipe Ramírez León3 and Kai-Uwe Lewandrowski4*
- 1Professor of Anesthesiology, Faculty of Medicine of Ribeirão Preto, University of São Paulo, Brazil
- 2MDFundación Universitaria Sanitas, Bogotá, Colombia, Research Team, Centro de Columna. Bogotá, Colombia, Centro de Cirugía de Mínima Invasión, CECIMIN-Clínica Reina Sofía, Bogotá, Colombia
- 3Orthopaedic Spine Surgeon, Director of Endoscopic Spine Clinic, Chile
- 4Center for Advanced Spine Care of Southern Arizona and Surgical Institute of Tucson, USA
Received: September 21, 2020; Published: October 20, 2020
Corresponding author: Kai-Uwe Lewandrowski, MD, Center for Advanced Spine Care of Southern Arizona and Surgical Institute of Tucson, Tucson AZ, USA
DOI: 10.32474/GJAPM.2020.03.000174
Abstract
Background: Providing adequate anesthesia that caters to rapid turn-over in an outpatient ambulatory surgery center setting is sometimes as much of an art as it is a science. Outpatient spine surgery is characterized by shorter simplified versions of their inpatient counterparts carried out in a hospital setting.
Objective: The monitored anesthesia care (MAC) and deep sedation with a laryngeal mask airway (LMA) in the prone position have received little attention in the anesthesia and spine surgery literature. The authors reviewed their clinical outcomes with the outpatient endoscopic spinal surgery in conjunction with these two types of anesthesias.
Methods: We performed an analysis of perioperative anesthesia and clinical outcomes in 184 patients who underwent lumbar endoscopic spinal surgery in two outpatient ambulatory surgery centers. The 184 patients consisted of 90 (48.9%) women and 94 (51.1%) men with an average age of 54.3 ± 15.4 years. The average follow-up of 43.27 months. The primary clinical outcome measures were the modified Macnab criteria. Chi-square testing was employed to analyze statistically significant associations between the type of anesthesia, preoperative diagnosis, the surgical level(s), and surgeon requirements for MAC versus balanced general anesthesia.
Results: At the final follow-up, the majority of patients had Excellent (93/184; 50.5%) and Good (74/184; 40.2%) Macnab outcomes regardless of treatment and anesthesia. There was minimal blood loss in all patients, and there were no complications such as dural tears, or hematomas. There were also no wound problems such as bleeding, or leaking of endoscopic irrigation fluid from the wound. Of the 184 study patients, 40 patients (21.7%) had a postoperative irritation of the dorsal root ganglion (DRG). The mean anesthesia time was 37 ± 9 minutes in patients under balanced general anesthesia. with LMA and 48 ± 12 minutes in MAC patients. All of the 184 patients were discharged under one hour from the PACU after an uneventful wakeup. Only six patients (3.26%) had postoperative nausea.
Conclusions: Monitored anesthesia care with sedation in an ambulatory surgery center in the prone position is best suited for endoscopic spinal decompressions. Intubation of the patient with general anesthesia is generally not required. Most patients can be managed with a nasal cannula, face mask, or a balanced general anesthesia with a laryngeal mask airway (LMA).
Keywords: Lumbar endoscopic decompression surgery; anesthesia management; clinical outcomes
Abbreviations: MAC: Monitored Anesthesia Care; LMA: Laryngeal Mask Airway; MISS: Minimally Invasive Spine Surgery; ASC: Ambulatory Surgery Center
Introduction
Surgical management has traditionally been accomplished via open approaches. Minimally Invasive Spine Surgery (MISS) is gaining popularity in the last decade. It has been the technique of choice for managing many degenerative diseases of the spine [1-3]. In the spine, the indications for MISS are mainly herniated intervertebral disc [1-6]. Compared to the traditional open spine surgery MISS has many advantages in the perioperative period, such as minimal tissue trauma, reduced blood loss, decreased postoperative pain, and a diminished need for deep anesthesia [5- 8]. Many patients experience a much shorter length of inpatient stay. 9 In endoscopic spine surgery, light sedation monitored anesthesia care is the critical element in allowing patients to discharge early from the recovery room or the ambulatory surgery center (ASC) [9, 10]. The authors describe their methodology of providing anesthesia tailored to the outpatient endoscopic decompression surgery, where patients not only need minimal amounts of sedatives and narcotics to feel comfortable throughout different stages of the spinal endoscopy surgery, are not intubated, and a rapidly recovered to be sent home from the recovery room typically within one hour from the operation. Depending on surgeon skill level, clinical indication, and type of endoscopic surgery there may be a need to keep the patient responsive where surgeon heavily rely on the patient’s verbalization ability during the procedure. The awake patient able to speak during operation with the surgeon is the most reliable and best monitoring of the patient’s neurological function during this delicate and potentially dangerous procedure. Surgeon with high skill level and a great deal of experience may not require a responsive patient. In such experienced hands, the surgery may be executed faster with the patient being under deep sedation throughout the entire endoscopic surgery. The anesthesiasurgeon author teams of this article have worked together for years. The developed protocols presented herein were developed in close communication between the physicians involved. In purpose of this article, is to summarize the anesthesia requirements for the endoscopic spinal surgery, the authors preferred airway management in the supine and prone position in an ambulatory surgery center, and the clinical outcomes they have achieved with their clinical program.
Materials and Methods
Patients
All patients in this case series suffered from sciatica-type low back and leg pain with claudication symptoms due to a contained lumbar disc herniation contributing to stenosis in the lateral recess, or extruded migrated disc herniations. This retrospective study selected from groups of consecutive patients seen in clinics of the two participating study sites. All patients provided informed consent. The total study population consisted of 184 patients 90 (48.9%) of which were female and 94 (51.1%) were male. Patients were matched to age, gender, and diagnosis. Patient enrollment at the study sites took place between 2012 and 2018. The mean follow-up was 43.27 months ranging from 24 to 111 months. The patients’ age ranged from 19 to 88 years, with a mean age of 54.3 years (standard deviation [SDV] = 15.4 years) with a normal age distribution (Figures 1,2). The inclusion and exclusion criteria for this study have been published elsewhere in detail and are briefly described in the following [8,11].
Figure 1: Age Distribution of the 184 study patients with the superimposed expected normal distribution (black line). Patient’s age ranged from 19 to 88 years of age and averaged 54.31 years.
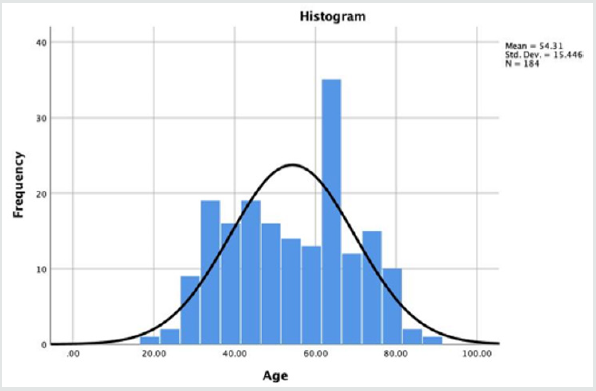
Figure 2: The quantile-quantile plot of the endoscopy patients’ age shows normal distribution. The average age was 54.31 ± 15.4 years ranging from 19 to 88 years.
Inclusion/Exclusion Criteria
The history, physical examination, the findings of the advanced preoperative imaging studies were recorded. Only patients suffering from sciatica-type back and leg pain due to foraminal HNP or lateral recess stenosis who failed non-operative treatment for a minimum of 12 weeks were included in this study. The size and location of the compressive pathology, whether from disc herniations, or other types of soft tissue or bony stenosis in the spinal canal, lateral recess, and neuroforamen were classified according to well-established radiographic classification systems [12-14]. Diagnostic selective nerve root blocks were used to determine the symptomatic painful level.28-35 Exclusion of patients from the study was prompted by a concurrent diagnosis of infection, tumor or metastatic disease, overt spondylolisthesis with more than 3 mm of translational motion on dynamic extension/flexion views, and claudication due to severe central (< 100 mm2) at the surgical level(s) [15].
Anesthesia For Spinal Endoscopy In Ambulatory Surgery Center (ASC)
The authors’ did not employ general anesthesia for endoscopic spine surgery in ASC to facilitate rapid recovery and discharge to home. Therefore, this team of authors typically decides for conscious sedation under monitored anesthesia care (MAC) [10,16] or balanced general anesthesia with a laryngeal mask airway (LMA) to perform the endoscopic surgery employing the Ramsay Sedation Scale (Figure 3) [17-21]. For MAC, the target was level 3 (patient able to respond to commands). The ventilation was found to be adequate at this level, and typically only a supplementary oxygen nasal cannula was needed. When communication with the patient was deemed not to be necessary, the anesthesiologist on this team of authors aimed for deep sedation Ramsay stage 5 (patient exhibits a sluggish response to light glabellar tap or loud auditory stimulus) When communication with the patient was not requested, balanced general anesthesia with LMA was employed regardless of supine or prone position. The following sedatives were employed:
Benzodiazepines: Diazepam, midazolam, and lorazepam are of the same pharmacological group [22-24]. All of them have active metabolites and can provoke agitation in older people. Midazolam was employed in ASC in low doses [25,26]. Since benzodiazepines are metabolized in the liver. Hence, the authors avoided it in patients with low hepatic function. The new alternative benzodiazepine, remimazolam, undergoes rapid metabolism by plasma and tissue esterases, so it is independent of the liver or kidneys. Midazolam is the most popular drug, but considering it has a relatively long half-life - t1/2ke0 - the average time to the drug is achieving the same concentration in the brain as in the plasma is estimated to be 9 min. [14] The anesthetists considered this pharmacokinetic dynamic when a second dose was used to avoid complications such as hypoventilation, airway obstruction, hypoxia, and hypotension [27].
Figure 3: Preoperative sagittal (a) and axial (b) T2-weighted MRI images of a 39-year old female patient undergoing endoscopic transforaminal decompression of the L4/5 level on the left side for symptomatic lateral recess stenosis due to disc herniation with an annular tear under MAC with local anesthesia. The surgeons requested verbal communication with the patient. Therefore, her feet were exposed (c) so the surgeon could assess motor function during the endoscopic decompression when neural elements could be at risk (d). The decompressed traversing L5 nerve root is shown in panel (e).

Propofol: Nowadays, propofol is the most intravenous drug used in short duration procedures because of its rapid onset and offset time [28-30]. The pain on injection can be easily overcome by adding a little amount of lidocaine to the mixture. It has a narrow therapeutic window, and patients may quickly go into hypotension and respiratory depression at plasma concentrations similar to those needed for ambulatory surgery sedation [31]. Hypotension may quickly develop because of its vasodilative properties [28]. Intravenous volume replenishment and vigilant monitoring of the patient’s vital signs are critical when using propofol. With the patient in the prone position with just a nasal cannula for continuous oxygen supply, the patient should not receive an intravenous dose of propofol higher than the recommended 1mg/kg IV. The continuous infusion was established at about 50 μg/kg/min [32]. When using the target-controlled infusion (TCI) technology, the aim was to maintain at 1 μg/mL concentration in the plasma, but it can vary depending on the respiratory drive. The anesthesiologist always used his clinical judgment when assessing the patients’ anxiety and level of pain control and sedation while safely providing comfort during the endoscopic procedure on the cervical spine. The latter was of particular importance when recognizing the patients’ ability to metabolize sedatives and narcotics rapidly. Particular attention was be taken in a narcotic-naive patient who may be easily deeply sedated.
Dexmedetomidine: Dexmedetomidine is an α-2-adrenoceptor agonist with sedative, anxiolytic, sympatholytic, and analgesicsparing effects while causing minimal depression of respiratory function (Figure 1) [33-36]. Hemodynamic effects include transient hypertension, bradycardia, and hypotension. 34Dexmedetomidine exerts its hypnotic action by activating alfa-2 receptors in the locus coeruleus, inducing unconsciousness similar to natural sleep, and the patient remains easily rousable and cooperative [35,37]. Dexmedetomidine is rapidly distributed and is mainly hepatic metabolized into inactive metabolites [38]. Typically, during intravenous infusion, our anesthesiologists used an induction dose of 1μg/kg for 10 min and a maintenance dose of 0.5 μg/kg/h. This dose was adjusted by the patient’s response, up to 1 μg/kg /h. [35] When administering MAC, dexmedetomidine was effectively used for maintaining the patient’s baseline sedation. Complementary propofol and remifentanil was employed as needed during the more painful parts of the endoscopic cervical decompression surgery [35]. Judicious application of local anesthesia with 1% lidocaine by the surgeon allowed also significantly improve patient comfort and decrease the need for systemic drug application.
Remifentanil: Remifentanil is a short-acting synthetic opioid, it is a μ-receptor agonist, rapidly hydrolyzed by plasma and tissue esterases. It does not accumulate with a prolonged infusion. Its context-sensitive half-life is +/- 4 min [37-41]. The combination of drugs with potent respiratory depressant properties can lead to a rapid compromise of respiration. Usually, the remifentanil dose was around 0,05 μg/kg /min when propofol is used concomitantly [33]. As the t1/2ke0 of remifentanil is 1.3 min, it is easily titratable [35]. This pharmacokinetic and pharmacodynamic profile suggests that remifentanil will be useful in situations where a rapidly titratable potent opioids effect was desirable during the endoscopic spinal surgery with a predictable offset of action without prolonged respiratory depression [32]. The anesthesiologist should always keep in mind that remifentanil has a small central volume of distribution. Therefore, it was not practical in bolus application and should only be employed by pump infusion. It was highly suitable for outpatient spine surgery [36].
Ketamine: Ketamine has a carbon chiral and can be found in both S(+) and R(-) enantiomers. The S(+) isomer is four times as potent as the R(-) counterpart. In the United States, ketamine is only available in the racemic mixture. In South America, the S(+) Ketamine, named dextroketamine, is currently in clinical use. Ketamine as mono-anesthetic has limited use because of hallucinations it can produce postoperatively. It usually is used in conjunction with other drugs with sedative effects [38-43]. One of the most beneficial ketamine properties is that it provides a high level of analgesia without respiratory depression [41]. The lack of respiratory depression is useful in prone patients such as those undergoing posterior cervical endoscopy [44]. The dose to achieve analgesia is minimal - 0.2-0.5 mg/kg -was typically sufficient. Employing ketamin was of real interest when the patient was obese, asthmatic, or had sleep apnea. The mixture of propofol and ketamine, known as ketofol, was used [20,45]. The ratio customarily used for these two drugs is 1:1 to 1:10.9 The team was aware that the recovery times of patients treated with ketofol may be longer than those of patients receiving propofol and fentanyl [39]. The use of ketamine is useful for better analgesia, which in low doses may be safely employed during spinal endoscopy in the prone position.
Endoscopic Surgical Technique
The surgeons of this study employed the transforaminal “outside-in” technique [29]. Serial dilation and foraminoplasty was employed to place the working cannula regardless of whether the “inside-out” or “outside-in” technique was followed. A foraminoplasty was performed were needed with power drills, trephines, chisels, and rongeurs following published techniques [4,8,11-13,29,30]. If bleeding occurred, a radiofrequency probe (Elliquence®) was used for coagulation [31]. The endoscopic decompression procedure was directly visualized throughout the surgery.
Clinical Follow-Up
Patients postoperative recovery was assessed by recording the time from arrival in the postanesthesia recovery unit (PAC) to discharge from the ambulatory surgery center – the PACU time. Any postoperative sequelae including náusea, vomiting, urinary retention, dysphoric wake-up, and excessive postoperative surgical pain requiring additional narcotic pain medication administration in the PACU were recorded. Primary measures of surgical outcomes were assessed employing the modified Macnab criteria [32,33]. All patients were instructed to be seen in follow-up for examination and management of any problems at 2 and 6 weeks and then at 3, 6, 12, and 24 months postoperatively. Unplanned visits to the emergency room, hospital admissions, or unforeseen postoperative problems or complications were recorded.
Correlative Surgical Outcomes Analysis
Statistical analysis of the PACU time and the primary clinical outcome measures, descriptive statistics (mean and standard deviation), crosstabulation statistics and measures of association were computed for two-way tables using IBM SPSS Statistics software, Version 26.0. The Pearson 2 and the likelihood-ratio 2 tests were used as statistical measures of association. Expected cell counts, continuity corrections, and likelihood ratios were calculated for some analyses. The confidence intervals (95%) for the likelihood ratios were calculated using the “log method” according to Altman et al. [36,37].
Result
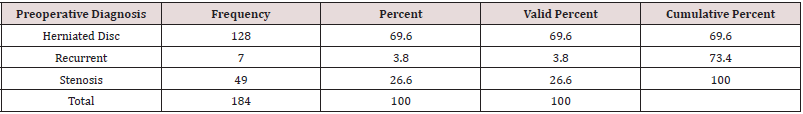
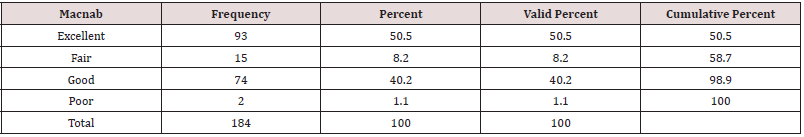
As expected, the 184 endoscopic spinal decompressions patients were mostly performed at the L4/5 (91/184; 49.5%), followed by L5/S1 (49/184; 26.6%), and two-level surgery from L4 to S1 (18/184; 9.8%; Table 1), respectively. The most common indication for surgery was herniated disc (128/184; 69.6%), followed by spinal stenosis (49/184; 26.6%), and recurrent herniation (7/184; 3.8%; Table 2). The anestesia times were consistently under one hour in all patients who underwent balanced general anesthesia with LMA (mean 37 ± 9 minutes). MAC patients had slightly longer anestesia times but there was no statistically significant difference between MAC- and deep sedation patients (mean 48 ± 12). All of the 184 patients, were discharged under one hour from the PACU after an uneventful wakeup. Only 6 patients (3.26%) suffered from a brief episode of postoperative nausea. There were no complications such as dural tears, or hematomas. There were also no wound problems such as bleeding, or leaking of endoscopic irrigation fluid from the wound. Of the 184 study patients, 40 patients (21.7%) had a postoperative irritation of the dorsal root ganglion (DRG). The remaining three-thirds of the study patients (144; 78.3%) had an entirely uneventful postoperative recovery without any problems in the postoperative recovery period. Cross-tabulating presence or absence of postoperative nausea did not eveal any statistical association with any particular sedative because of the low numbers. There were no readmission to an emergency room for management of any postoeprative sequelae or complications. All patients were managed postoperatively in an office setting according to the postoperative follow-up schedule. At final followup, the majority of patients had Excellent (93/184; 50.5%) and Good (74/184; 40.2%) Macnab outcomes regardless of treatment (Table 3). Fair (15/184;8.2%) and Poor (2/184;1.1%) results were achieved in the remaining 9.3% of patients.
Table 1: Frequency distribution of lumbar surgical levels treated with endoscopic spinal surgery (n = 184).
Discussion
Recovery from anesthesia and surgery is typically faster in ASC
the sooner the patient can be sent home. Patient satisfaction has
consistently been reported higher when recovery from a surgical
procedure takes place at home [46-48]. Therefore, the authors
attempted to demystify the belief that spinal surgery is always
complicated, and does need to take place in a hospital under
general anesthesia requiring several days of admission to recover
from extensive surgery with significant blood loss. In comparision,
the majority of spinal endoscopies are percutaneous posterior
procedures with neglible blood loss, minimal muscle dissection,
and hardly any postoperative wound pain. These posterior
transforaminal or interlaminar decompression procedures seem
preferred by most endoscopic spine surgeons for greater versatility,
simplicity, and lower risks to the vital structures in the spine [49-58].
For most anesthesiologists, prone position calls for endotracheal
intubation and general anesthesia to secure the airway throughout
the surgery adequately [59,60]. While endotracheal intubation with
general anesthesia is useful in quickly correcting intraoperative
respiratory depression, or even maintain the patient’s mechanical
ventilation should the patient cease to breathe spontaneously under
overzealous use of sedation, it certainly prolongs the postoperative
recovery. Second, general anesthesia endotracheal intubation may
lead to other postoperative problems, including prolonged wakeup,
urinary retention with the need for catheterization in the recovery
room, cardiopulmonary compromise, constipation, etc. [61-72]
Hence, posterior and anterior endoscopic cervical spine surgeries
are preferrably carried out under a combination of local anesthesia
with sedation to minimize the risk of neural injury and streamline
recovery and workflow in an outpatient surgery center.
Sedation in ASC has to allow a patient to undergo a potentially
unpleasant and painful procedure by making it tolerable and
more comfortable to the patient. Sedation is a continuous process
of decreasing consciousness ranging from anxiolysis to a deep
state where nociception is significantly reduced [28]. For spinal
endoscopy, the anesthesiologist should aim for a short time onset
of sedation combined with a rapid and consistent recovery profile.
The sedative concentration should ideally not be associated with any adverse hemodynamics, respiratory, and metabolic
consequences. The sedative drugs most used in ambulatory
sedation were midazolam, propofol, ketamine, dexmedetomidine,
remifentanil, and fentanyl. There is a new benzodiazepine,
remimazolam, under investigation in phase three clinical trials. It
may be in clinical applications in the future. Comfortable position
of the patient on the operating table is often supervised by default
by the anesthesiologist and is relevant as to position the patient in
such way that allows best ventilation. Adequate and comfortable
position in the prone position on a lordotic frame or chest rolls is
of particular importance. Local anesthesia should be used by the
surgeon to anesthetize the entire surgical access corridor and the
surgical site, which in the case of cervical endoscopy entails skin
anesthesia, the paraspinal muscles, and the facet joint complex.
The concomitant use of long-acting local anesthetic may not
only diminish painful stimulus during surgery but also control
postoperative pain and avoid a dysphoric wake in the recovery
room. To achieve the right sedation level and comfort in the patient
undergoing spinal endoscopy, the anesthesiologist has to perceive
and anticipate situations where the patient may feel severe pain
and needs to be responsive and cooperative. Since the surgeon may
concentrate on the operation, the anesthesiologists should also rely
on assessing the stimulation level on his monitors.
Results of this consecutive case series corroborated the authors’ clinical experience, that anesthesia needs to be tailored around the goals of the surgery. In outpatient endoscopic spine surgery, short and simplified anterior and posterior minimally invasive procedures are commonly performed. These include posterior foraminotomies, laminotomies, or partial laminectomies. The incidence of postoperative nausea was 3.26% and very low compared to rates reported with inpatient spine surgery under general anesthesia endotracheal intubation [73-76]. The surgery times were also shorter than reported with inpatient open lumbar decompression surgery [76]. Cliincial outcomes with the decompression procedure were on par with previously reported outcomes [77-80]. The postoperative DRG irritations were essentially the only sequela that bothered some 21.7% of study patients [81]. The remaining three quarters of enrolled study patients had an entirely uneventful recovery. Complications with the endoscopic spinal decompression procedure requiring additional aggressive aftercare are exceedingly uncommon [57,82]. These favorable clinical outcomes assessments provided by patients were found to be reflective of their experience with anesthesia at the ASC [83]. The anesthesia requirements may vary by anterior or posterior approach, the conjunctive use of local anesthesia by the surgeon, and the surgeon’s preference as to whether communication with the patient during surgery is desired. Using a face mask or nasal cannula for continuous oxygenation of the patient may be preferred in the latter scenario. In patients where that was not requested or required, the authors successfully used a laryngeal mask airway (LMA) as an adjunct to ventilation under balanced general anesthesia to avoid obstruction. In patients with medical comorbidities such as asthma, chronic obstructive pulmonary disease (COPD), cardiopulmonary disease, diabetes, or obesity, the LMA may improve ventilation and give the anesthesiologist more confidence in providing more profound anesthesia. As outlined in the various sedatives review, their pharmacokinetics-pharmacokinetics and dynamics may quickly take a patient from cooperative and responsive (Ramsay stage 2 to 3) to respond sluggishly or be entirely unresponsive to a stimulus (Ramsay Stage 5 and 6). Therefore, a combination of sedatives and anesthetics tailored to the stimulatioin level created by the endoscopic decompression procedure is the preferred course of action.
A combination regimen, multimodal sedation, using several sedatives is aimed at emphasizing the advantages of the various medications. For example, ketamine was combined with propofol because of its excellent analgesic effects without suppressing respiration. Ketofol is such a combination drug. However, the ketamine is associated with postoperative hallucinations, for which reason it has fallen out of favor and is less commonly employed in the ASC setting. Remifentanil is another narcotic that has been used in conjunction with propofol. Its rapid onset and offset of action have made a favorite amongst many anesthesiologists. However, the drug is expensive, and therefore its widespread use in ASC may be limited by its prohibitive cost. In some countries, the price is not that high because many laboratories are making it. Fentanyl may be used as an alternative to remifentanil by has a much longer halflife. Its routine use requires experience, and this team of authors recommends using it judiciously and only dose it before the painful portion of the cervical endoscopic decompression procedure. Dexmedetomidine is a newer drug that has found its way into ASC. The drug is highly attractive because of its excellent analgesic effects while causing minimal depression of respiratory function [84-87]. However, the drug may cause transient hypertension, bradycardia, and hypotension. Therefore, some anesthesiologists tend to stick with what they know best – a simple combination regimen of fentanyl and propofol – a time-proven and cost-effective solution to outpatient endoscopic sedation requirements cervical decompression surgery. In patients in whom an LMA has been placed, inhalation anesthesia with sevoflurane. at a fresh gas flow rate of 1 L/min in adults, the recommended mean maximum concentrations in the anesthesia circuit are approximately 20 ppm (0.002%) with soda-lime and 30 ppm (0.003%) with Baralyme.
In summary, close communication between the surgeon and the anesthesiologist is recommended to tailor the anesthesia to minimize its side effects. Rapid discharge of patients from the ASC is common in many outpatient ambulatory surgery centers and even hospitals. Both rely on turning patients over efficiently yet safely to generate revenue. The conjunctive use of local anesthesia with sedation should further diminish the need for general anesthesia. Whether or not communication between the patient and the surgeon is required during the endoscopic spinal decompression surgery should be determined by the anesthesiologist before determining the appropriate choice of anesthesia. If cooperation by the patient is not needed, this team of authors recommends the deliberate use of balanced general anesthesia with an LMA as it improves ventilation and diminishes airway obstruction problems in the prone position. Patient comfort during spinal endoscopy provided by adequate analgesia and sedation allows the surgeon to execute the endoscopic surgery expeditiously and promptly, which reduces the need for sedatives and improves recovery. Anesthesiologistsurgeon teams that worked together consistently for a long time typically achieve the best patient satisfaction with the anesthesia for patients who undergo spinal endoscopy in an outpatient ASC setting. Both parties know each other’s “habits” to a point where it becomes second nature. Therefore, the authors recommend that such joint anesthesia –surgery protocols are written out and communicated to all relevant staff members involved in the patient’s perioperative- and postoperative care. Such protocols will likely guarantee consistently better clinical outcomes with high patient satisfaction [88-90].
Conclusion
Monitored anesthesia care or deep sedation in an ambulatory surgery center in the prone position is best suited for endoscopic spinal decompressions. Endotracheal intubation of the patient with general anesthesia is generally not required. Most patients can be managed with a nasal cannula, face mask, or a laryngeal mask airway (LMA). Typically, these airway management strategies work well for patients placed in the supine or the prone position. Outpatient endoscopic surgeries on the lumbar spine are usually shorter than there impatient open surgery counterparts. Therefore, patients who have complex medical comorbidities requiring intubation should be considered for surgery in a hospital or clinical setting where providers are set up for longer recovery if the patient requires postoperative mechanical ventilation. Although these scenarios are uncommon, both surgeon- and anesthesia teams should avoid complacency in selecting patients for outpatient endoscopic spine surgery under local anesthesia and sedation. Every patient should be vetted preoperatively by employing a robust checklist-type protocol to prevent cancellation of surgery in the preoperative holding area, and prolonged wake up in the recovery room or transfer to a tertiary medical facility for further definitive medical management.
Conflicts of Interest
No direct, or indirect conflicts of interest.
Disclaimer
The views expressed in this article represent those of the authors and no other entity or organization.
Conflicts
This manuscript is not meant for or intended to push any other agenda other than reporting the clinical outcome data following endoscopic spinal decompression for sciatica-type back and leg pain. The motive for compiling this clinically relevant information is by no means created and/or correlated to directly enrich anyone due to its publication. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Adamson TE (2004) The impact of minimally invasive cervical spine surgery. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine 1: 43-46.
- Skovrlj B and Qureshi SA (2017) Minimally invasive cervical spine surgery. J Neurosurg Sci 61: 325-334.
- Rubino F, Deutsch H, Pamoukian V, J F Zhu, W A King, et al. (2000) Minimally invasive spine surgery: an animal model for endoscopic approach to the anterior cervical and upper thoracic spine. J Laparoendosc Adv Surg Tech A 10: 309-313.
- Fessler RG, O'Toole JE, Eichholz KM, Mick J Perez-Cruet (2006) The development of minimally invasive spine surgery. Neurosurg Clin N Am 17: 401-409.
- McClelland S, Goldstein JA (2007) Minimally Invasive versus Open Spine Surgery: What Does the Best Evidence Tell Us? J Neurosci Rural Pract 8: 194-198.
- Patel PD, Canseco JA, Houlihan N, Alyssa Gabay, Giovanni Grasso, et al. (2020) Overview of Minimally Invasive Spine Surgery. World Neurosurg 142: 43-56.
- Jan-Helge Klingler, Ronen Sircar, Christian Scheiwe, Evangelos Kogias, Florian Volz, et al. (2017) Comparative Study of C-arms for Intraoperative 3-dimensional Imaging and Navigation in Minimally Invasive Spine Surgery Part I: Applicability and Image Quality. Clin Spine Surg 30: 276-284.
- Afolabi A, Weir TB, Usmani MF, Jael E Camacho, Jacob J Bruckner, et al. (2020) Comparison of percutaneous minimally invasive versus open posterior spine surgery for fixation of thoracolumbar fractures: A retrospective matched cohort analysis. J Orthop 18: 185-190.
- Ghisi D, Fanelli A, Tosi M, M Nuzzi, G Fanelli, et al. (2005) Monitored anesthesia care. Minerva Anestesiol 71: 533-538.
- Berkenstadt H, Perel A, Hadani M, I Unofrievich, Z Ram (2001) Monitored anesthesia care using remifentanil and propofol for awake craniotomy. J Neurosurg Anesthesiol 13: 246-249.
- Allen SM, Madrio ME (2019) Ramsay Sedation Scale Project: Small, Easy Changes for a Big Effect on Patient Safety. Crit Care Nurse 39: 64-66.
- Lewis BS, Shlien RD, Wayne JD, Richard J Knight, Robert A Aldoroty (1989) Diazepam versus midazolam (versed) in outpatient colonoscopy: a double-blind randomized study. Gastrointest Endosc 35: 33-36.
- Avramov MN, Smith I and White PF (1996) Interactions between midazolam and remifentanil during monitored anesthesia care. Anesthesiology 85: 1283-1289.
- Taylor E, Ghouri AF and White PF (1992) Midazolam in combination with propofol for sedation during local anesthesia. J Clin Anesth 4: 213-216.
- Gold MI, Watkins WD, Sung YF, J Yarmush, F Chung, et al. (1997) Remifentanil versus remifentanil/midazolam for ambulatory surgery during monitored anesthesia care. Anesthesiology 87: 51-57.
- Gelfman SS, Gracely RH, Driscoll EJ, P R Wirdzek, J B Sweet, et al. (1978) Conscious sedation with intravenous drugs: a study of amnesia. J Oral Surg 36: 191-197.
- Barends CRM, Absalom AR, Struys M (2018) Drug selection for ambulatory procedural sedation. Curr Opin Anaesthesiol 2018; 31: 673-678.
- Kwak HJ, Kim JY, Kim YB, Chae JY Kim (2008) The optimum bolus dose of remifentanil to facilitate laryngeal mask airway insertion with a single standard dose of propofol at induction in children. Anaesthesia 63: 954-958.
- Hertzog JH, Campbell JK, Dalton HJ, G J Hauser (1999) Propofol anesthesia for invasive procedures in ambulatory and hospitalized children: experience in the pediatric intensive care unit. Pediatrics 103: E30.
- Frey K, Sukhani R, Pawlowski J, A L Pappas, M Mikat-Stevens, et al. (1999) Propofol versus propofol-ketamine sedation for retrobulbar nerve block: comparison of sedation quality, intraocular pressure changes, and recovery profiles. Anesth Analg 89: 317-321.
- Klein SM, Hauser GJ, Anderson BD, Aziza T Shad, Joseph E Gootenberg, et al. (2003) Comparison of intermittent versus continuous infusion of propofol for elective oncology procedures in children. Pediatr Crit Care Med 4(1): 78-82.
- Sukhani R, Lurie J and Jabamoni R (1994) Propofol for ambulatory gynecologic laparoscopy: does omission of nitrous oxide alter postoperative emetic sequelae and recovery? Anesth Analg 78: 831-835.
- Uusalo P, Guillaume S, Siren S, Tuula Manner, Sanna Vilo, et al. (2020) Pharmacokinetics and Sedative Effects of Intranasal Dexmedetomidine in Ambulatory Pediatric Patients. Anesth Analg 130: 949-957.
- Kumari A, Singh AP, Vidhan J, Ruchi Gupta, Jonny Dhawan, et al. (2018) The Sedative and Propofol-Sparing Effect of Dexmedetomidine and Midazolam as Premedicants in Minor Gynecological Day Care Surgeries: A Randomized Placebo-Controlled Study. Anesth Essays Res 12(2): 423-427.
- Riefkohl R, Cole NM, Cox EB (1984) The effectiveness of benzodiazepines and narcotics in outpatient surgery. Aesthetic Plast Surg 8: 227-230.
- Garcia-Pedrajas F, Monedero P (1992) Benzodiazepines in anesthesiology. Clinical applications (II)]. Rev Esp Anestesiol Reanim 39: 126-131.
- Loeffler PM (1992) Oral benzodiazepines and conscious sedation: a review. J Oral Maxillofac Surg 50: 989-997.
- Lepresle E, Debras C (1983) Use of benzodiazepines in anesthesia and resuscitation. Encephale 9: 267B-271B.
- Long K, Ruiz J, Kee S, Alicia Kowalski, Farzin Goravanchi, et al. (2016) Effect of adjunctive dexmedetomidine on postoperative intravenous opioid administration in patients undergoing thyroidectomy in an ambulatory setting. J Clin Anesth 35: 361-364.
- Das A, Dutta S, Chattopadhyay S, S Chhaule, T Mitra, et al. (2016) Pain relief after ambulatory hand surgery: A comparison between dexmedetomidine and clonidine as adjuvant in axillary brachial plexus block: A prospective, double-blinded, randomized controlled study. Saudi J Anaesth 10: 6-12.
- Weerink MAS, Struys M, Hannivoort LN, Clemens R M Barends, Anthony R Absalom et al. (2017) Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin Pharmacokinet 56: 893-913.
- Wu JX, Assel M, Vickers A, Anoushka M Afonso, Rebecca S Twersky, et al. (2019) Impact of intraoperative remifentanil on postoperative pain and opioid use in thyroid surgery. J Surg Oncol 120: 1456-1461.
- Torun AC, Yilmaz MZ, Ozkan N, B Ustun, E Koksal, et al. (2017) Sedative-analgesic activity of remifentanil and effects of preoperative anxiety on perceived pain in outpatient mandibular third molar surgery. Int J Oral Maxillofac Surg 46: 379-384.
- Sklika E, Kalimeris K, Perrea D, Nikolaos Stavropoulos, Georgia Kostopanagiotou, et al. (2016) Remifentanil Vs Fentanyl during Day Case Dental Surgery in People with Special Needs: A Comparative, Pilot Study of Their Effect on Stress Response and Postoperative Pain. Middle East J Anaesthesiol 23: 509-515.
- Sclar DA (2015) Remifentanil, fentanyl, or the combination in surgical procedures in the United States: predictors of use in patients with organ impairment or obesity. Clin Drug Investig 35(1): 53-59.
- Hara R, Hirota K, Sato M, Hiroko Tanabe, Tomoko Yazawa, et al. (2013) The impact of remifentanil on incidence and severity of postoperative nausea and vomiting in a university hospital-based ambulatory surgery center: a retrospective observation study. Korean J Anesthesiol 65: 142-146.
- Chillemi S, Sinardi D, Marino A, G Mantarro, R Campisi (2002) The use of remifentanil for bloodless surgical field during vertebral disc resection. Minerva Anestesiol 68: 645-649.
- Garg K, Grewal G, Grewal A, Avtar Singh, Atul Mishra, et al. (2014) Hemodynamic responses with different dose of ketamine and propofol in day care gynecological surgeries. J Clin Diagn Res; 7(11): 2548-2550.
- Kramer KJ, Ganzberg S, Prior S, Robert G Rashid (2012) Comparison of propofol-remifentanil versus propofol-ketamine deep sedation for third molar surgery. Anesth Prog 59(3): 107-117.
- Cillo JE, Jr (2012) Analysis of propofol and low-dose ketamine admixtures for adult outpatient dentoalveolar surgery: a prospective, randomized, positive-controlled clinical trial. J Oral Maxillofac Surg 70: 537-546.
- Aydin ON, Ugur B, Ozgun S, Eyigor H, Copcu O, et al. (2007) Pain prevention with intraoperative ketamine in outpatient children undergoing tonsillectomy or tonsillectomy and adenotomy. J Clin Anesth 19(2): 115-119.
- White M, de Graaff P, Renshof B, E. van Kan, M. Dzoljic (2006) Pharmacokinetics of S(+) ketamine derived from target controlled infusion. Br J Anaesth 96(3): 330-334.
- Dalsasso M, Tresin P, Innocente F, Veronese S, Ori C (2005) Low-dose ketamine with clonidine and midazolam for adult day care surgery. Eur J Anaesthesiol 22(1): 67-68.
- Ersek RA (2004) Dissociative anesthesia for safety's sake: ketamine and diazepam--a 35-year personal experience. Plast Reconstr Surg 113(7): 1955-1959.
- Badrinath S, Avramov MN, Shadrick M, Witt TR, Ivankovichet AD, et al. (2000) The use of a ketamine-propofol combination during monitored anesthesia care. Anesth Analg 90: 858-862.
- Hersht M, Massicotte EM, Bernstein M (2007) Patient satisfaction with outpatient lumbar microsurgical discectomy: a qualitative study. Can J Surg 50: 445-449.
- Bible JE, Shau DN, Kay HF, Joseph S Cheng , Oran S Aaronson, et al. (2018) Are Low Patient Satisfaction Scores Always Due to the Provider?: Determinants of Patient Satisfaction Scores During Spine Clinic Visits. Spine (Phila Pa 1976) 43: 58-64.
- Johnson BC, Vasquez-Montes D, Steinmetz L, Aaron J Buckland, John A Bendo, et al. (2019) Association Between Nonmodifiable Demographic Factors and Patient Satisfaction Scores in Spine Surgery Clinics. Orthopedics 42: 143-148.
- Liu W, Li Q, Li Z, Lei Chen, Dasheng Tian, et al. (2019) Clinical efficacy of percutaneous transforaminal endoscopic discectomy in treating adolescent lumbar disc herniation. Medicine (Baltimore) 98(99): e14682.
- Yang J, Liu C, Hai Y, Peng Yin, Lijin Zhou, et al. (2019) Percutaneous Endoscopic Transforaminal Lumbar Interbody Fusion for the Treatment of Lumbar Spinal Stenosis: Preliminary Report of Seven Cases with 12-Month Follow-Up. Biomed Res Int 2019: 3091459.
- Yeung A, Roberts A, Zhu L, Lei Qi , Jun Zhang, et al. (2019) Treatment of Soft Tissue and Bony Spinal Stenosis by a Visualized Endoscopic Transforaminal Technique Under Local Anesthesia. Neurospine 16(1): 52-62.
- Katzell JL (2020) Risk factors predicting less favorable outcomes in endoscopic lumbar discectomies. J Spine Surg 6: S155-S164.
- Lewandrowski KU (2020) The strategies behind "inside-out" and "outside-in" endoscopy of the lumbar spine: treating the pain generator. J Spine Surg 6: S35-S39.
- Lewandrowski KU, Dowling A, de Carvalho P, André Luiz Calderaro 4, Thiago Soares Dos Santos, et al. (2020) Indication And Contraindication Of Endoscopic Transforaminal Lumbar Decompression. World Neurosurg S1878-8750(20): 30547-30547.
- Lewandrowski KU, Yeung A (2020) Lumbar Endoscopic Bony and Soft Tissue Decompression With the Hybridized Inside-Out Approach: A Review And Technical Note. Neurospine 17: S34-S43.
- Merkow J, Varhabhatla N, Manchikanti L, Alan D Kaye , Richard D Urman, et al. (2020) Minimally Invasive Lumbar Decompression and Interspinous Process Device for the Management of Symptomatic Lumbar Spinal Stenosis: a Literature Review. Curr Pain Headache Rep 24(4): 13.
- Telfeian AE, Shen J, Ali R, Adetokunbo Oyelese, Jared Fridley, et al. (2020) Incidence and Implications of Incidental Durotomy in Transforaminal Endoscopic Spine Surgery: Case Series. World Neurosurg 134: e951-e955.
- Wu PH, Kim HS, Lee YJ, Dae Hwan Kim, Jun Hyung Lee, et al. (2020) Uniportal Full Endoscopic Posterolateral Transforaminal Lumbar Interbody Fusion with Endoscopic Disc Drilling Preparation Technique for Symptomatic Foraminal Stenosis Secondary to Severe Collapsed Disc Space: A Clinical and Computer Tomographic Study with Technical Note. Brain Sci 10(6): 373.
- Sharma V, Verghese C, McKenna PJ (2010) Prospective audit on the use of the LMA-Supreme for airway management of adult patients undergoing elective orthopaedic surgery in prone position. Br J Anaesth 105(2): 228-232.
- Kang F, Li J, Chai X, JinGui Yu, Hua Ming Zhang, et al. (2015) Comparison of the I-gel laryngeal mask airway with the LMA-supreme for airway management in patients undergoing elective lumbar vertebral surgery. J Neurosurg Anesthesiol 27(1): 37-41.
- Yeung A, Lewandrowski KU (2020) Five-year clinical outcomes with endoscopic transforaminal foraminoplasty for symptomatic degenerative conditions of the lumbar spine: a comparative study of inside-out versus outside-in techniques. J Spine Surg 6(1): S66-S83.
- Yeung A, Wei SH (2020) Surgical outcome of workman's comp patients undergoing endoscopic foraminal decompression for lumbar herniated disc. J Spine Surg 6(1): S116-S119.
- Yin J, Jiang Y, Nong L (2020) Transforaminal approach versus interlaminar approach: A meta-analysis of operative complication of percutaneous endoscopic lumbar discectomy. Medicine (Baltimore) 99(25): e20709.
- Li Z, Long H, Huang F, Y Zhang, J Xu, et al. (2019) Impact of Epidural Versus General Anesthesia on Major Lumbar Surgery in Elderly Patients. Clin Spine Surg 32(1): E7-E12.
- Kolcun JPG, Brusko GD, Wang MY (2019) Endoscopic transforaminal lumbar interbody fusion without general anesthesia: technical innovations and outcomes. Ann Transl Med 7: 167.
- Hua W, Zhang Y, Wu X, Yong Gao, Shuai Li, et al. (2019) Full-Endoscopic Visualized Foraminoplasty and Discectomy Under General Anesthesia in the Treatment of L4-L5 and L5-S1 Disc Herniation. Spine (Phila Pa 1976) 44(16): E984-E991.
- Finsterwald M, Muster M, Farshad M, Saporito A, Brada M, et al. (2018) Spinal versus general anesthesia for lumbar spine surgery in high risk patients: Perioperative hemodynamic stability, complications and costs. J Clin Anesth 46: 3-7.
- Zorrilla-Vaca A, Healy RJ and Mirski MA (2017) A Comparison of Regional Versus General Anesthesia for Lumbar Spine Surgery: A Meta-Analysis of Randomized Studies. J Neurosurg Anesthesiol 29(4): 415-425.
- Apipan B, Rummasak D, Wongsirichat N (2016) Postoperative nausea and vomiting after general anesthesia for oral and maxillofacial surgery. J Dent Anesth Pain Med 16(4): 273-281.
- Ulutas M, Secer M, Taskapilioglu O, Karadas S, Akyimaz AA, et al. (2015) General versus epidural anesthesia for lumbar microdiscectomy. J Clin Neurosci 22(8): 1309-1313.
- Dagistan Y, Okmen K, Dagistan E, Guler A, Ozkan N (2015) Lumbar Microdiscectomy Under Spinal and General Anesthesia: A Comparative Study. Turk Neurosurg 25(5): 685-689.
- Vural C, Yorukoglu D (2014) Comparison of patient satisfaction and cost in spinal and general anesthesia for lumbar disc surgery. Turk Neurosurg 24(3): 380-384.
- De Rojas JO, Syre P and Welch WC (2014) Regional anesthesia versus general anesthesia for surgery on the lumbar spine: a review of the modern literature. Clin Neurol Neurosurg 119: 39-43.
- McLain RF, Tetzlaff JE, Bell GR, Kai Uwe-Lewandrowski, Helen J Yoon, et al. (2007) Microdiscectomy: spinal anesthesia offers optimal results in general patient population. J Surg Orthop Adv 16(1): 5-11.
- McLain RF, Bell GR, Kalfas I, John E Tetzlaff, Helen J Yoon (2004) Complications associated with lumbar laminectomy: a comparison of spinal versus general anesthesia. Spine (Phila Pa 1976) 29(22): 2542-2547.
- Jellish WS, Thalji Z, Stevenson K, Shea J (1996) A prospective randomized study comparing short- and intermediate-term perioperative outcome variables after spinal or general anesthesia for lumbar disk and laminectomy surgery. Anesth Analg 83(3): 559-564.
- Dowling A, Barcenas JGH, Lewandrowski KU (2020) Transforaminal endoscopic decompression and uninstrumented allograft lumbar interbody fusion: A feasibility study in patients with end-stage vacuum degenerative disc disease. Clin Neurol Neurosurg 196: 106002.
- Lewandrowski KU, PST DEC, P DEC, Yeung A (2020) Minimal Clinically Important Difference in Patient-Reported Outcome Measures with the Transforaminal Endoscopic Decompression for Lateral Recess and Foraminal Stenosis. Int J Spine Surg 14(2): 254-266.
- Lewandrowski KU, Ransom NA (2020) Five-year clinical outcomes with endoscopic transforaminal outside-in foraminoplasty techniques for symptomatic degenerative conditions of the lumbar spine. J Spine Surg 6: S54-S65.
- Lewandrowski KU, Ransom NA, Yeung A (2020) Return to work and recovery time analysis after outpatient endoscopic lumbar transforaminal decompression surgery. J Spine Surg 6: S100-S115.
- Lewandrowski KU, Dowling A, Calderaro AL, Santos TSD, Bergamaschi JPM, et al. (2020) Dysethesia due to irritation of the dorsal root ganglion following lumbar transforaminal endoscopy: Analysis of frequency and contributing factors. Clin Neurol Neurosurg 197: 106073.
- Lewandrowski KU (2019) Incidence, Management, and Cost of Complications After Transforaminal Endoscopic Decompression Surgery for Lumbar Foraminal and Lateral Recess Stenosis: A Value Proposition for Outpatient Ambulatory Surgery. Int J Spine Surg 13(1): 53-67.
- Menendez JY, Omar NB, Chagoya G, Tabibian BE, Elsayad GA, et al. (2019) Patient Satisfaction in Spine Surgery: A Systematic Review of the Literature. Asian Spine J 13(6): 1047-1057.
- Uusalo P, Guillaume S, Siren S, Tuula Manner, Sanna Vilo, et al. (2020) Pharmacokinetics and Sedative Effects of Intranasal Dexmedetomidine in Ambulatory Pediatric Patients. Anesth Analg 130: 949-957.
- Kumari A, Singh AP, Vidhan J, Ruchi Gupta, Jonny Dhawan, et al. (2018) The Sedative and Propofol-Sparing Effect of Dexmedetomidine and Midazolam as Premedicants in Minor Gynecological Day Care Surgeries: A Randomized Placebo-Controlled Study. Anesth Essays Res 12(2): 423-427.
- Long K, Ruiz J, Kee S, Alicia Kowalski, Farzin Goravanchi, et al. (2016) Effect of adjunctive dexmedetomidine on postoperative intravenous opioid administration in patients undergoing thyroidectomy in an ambulatory setting. J Clin Anesth 35: 361-364.
- Das A, Dutta S, Chattopadhyay S, S Chhaule, T Mitra, et al. (2016) Pain relief after ambulatory hand surgery: A comparison between dexmedetomidine and clonidine as adjuvant in axillary brachial plexus block: A prospective, double-blinded, randomized controlled study. Saudi J Anaesth 10: 6-12.
- Mikhael MM, Celestre PC, Wolf CF, Tom E Mroz, Jeffrey C Wang. (2012) Minimally invasive cervical spine foraminotomy and lateral mass screw placement. Spine (Phila Pa 1976) 37: E318-322.
- Uusalo P, Guillaume S, Siren S, Tuula Manner, Sanna Vilo, et al. (2020) Pharmacokinetics and Sedative Effects of Intranasal Dexmedetomidine in Ambulatory Pediatric Patients. Anesth Analg 130: 949-957.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...